The brilliant colors of a soap film are directly related to the film’s thickness. Black regions, like the one in the upper right of this image, are the thinnest regions and may be less than 100 nanometers thick. (That’s smaller than the shortest wavelength of visible light!) The colors of the peacock-feather-like blooms along the bottom of the image demonstrate significant variations in film thickness. This is caused by uneven concentrations of surfactants in the film. The variations in concentration causes differences in local surface tension, which in turn moves fluid around within the film. This is known as a Marangoni effect. (Image credit: S. Berg and S. Troian)
Tag: marangoni effect

Deforming Soap Films
It’s the time of year when new Gallery of Fluid Motion videos start popping up online. We’ve already featured several and no doubt there will be more to come. Today’s post is a submission from Saad Bhamla, who gave this introduction to the work:
Soap bubbles occupy the rare position of delighting and fascinating both young children and scientific minds alike. Sir Isaac Newton, Joseph Plateau, Carlo Marangoni and Pierre-Gilles de Gennes, not to mention countless others, have discovered remarkable results in optics, molecular forces and fluid dynamics from investigating this seemingly simple system.
This video is a compilation of curiosity-driven experiments that systematically investigate the surface flows on a rising soap bubble. From childhood experience, we are familiar with the vibrant colors and mesmerizing display of chaotic flows on the surface of a soap bubble. These flows arise due to surface tension gradients, also known as Marangoni flows or instabilities. In this video, we show the surprising effect of layering multiple instabilities on top of each other, highlighting that unexpected new phenomena are still waiting to be discovered, even in the simple soap bubble.
As illustrated in the video, raising a bubble beneath the soap film moves surfactants in the film, which causes local differences in surface tension. Any time a difference in surface tension exists, fluid will flow from areas of low surface tension to ones with higher surface tension. This is called the Marangoni effect. On a soap bubble, this is visible in the chaotic swirling colors we see. In this system, Bhamla and his co-author found that by raising the bubble in steps, they could “freeze” the Marangoni-induced patterns created by the previous motion. (Video credit and submission: S. Bhamla et al.)

Healing Soap Films
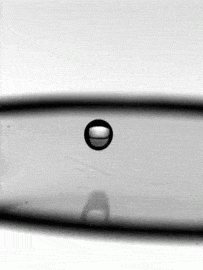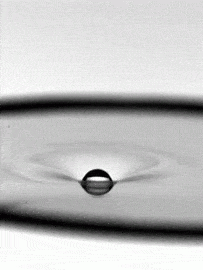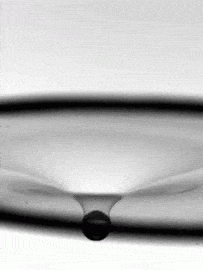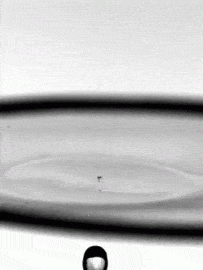
As fragile as a soap bubble seems, these films have remarkable powers of self-healing. The animation above shows a falling water droplet passing through a soap film without bursting it. An important factor here is that the water droplet is wet–passing a dry object through a soap film is a quick way to burst it, as those who have played with bubbles know. The droplet’s inertia deforms the soap film, creating a cavity. If the drop’s momentum were smaller, the film could actually bounce the droplet back like a trampoline, but here the droplet wins out. The film breaks enough to let the drop through, but its cavity quickly pinches off and the film heals thanks to the stabilizing effect of its soapy surfactants. (Image credit: H. Kim, source)

“Jack and the Giant”
This fantastic music video by Kim Pimmel is a beautiful merger of art and fluid dynamics. Using household goods (and some slightly more exotic ferrofluid), the video shows how mesmerizing diffusion, buoyancy, Marangoni flow, and other fluid effects can be up close. It may also be the first time I’ve ever seen fluid dynamics–specifically bubbles–used as characters! Also be sure to check out some of his previous videos, many of which also feature cool fluid dynamics. (Video credit and submission: K. Pimmel)

The Marangoni Effect
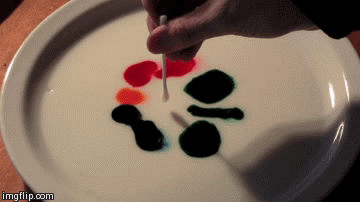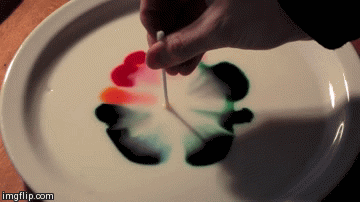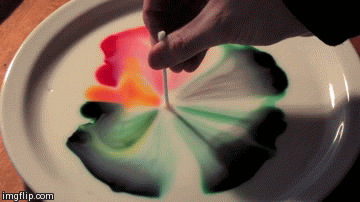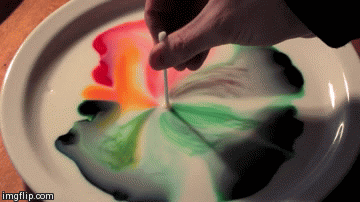
Differences in surface tension can create Marangoni flow along an interface. Imagine a shallow bowl filled with a liquid. In the middle of the fluid, every molecule is surrounded on all sides by like molecules, which push and pull it equally in all directions. But at the surface, the fluid molecules are only acted on by similar molecules in some directions. This imbalance in molecular forces is what creates surface tension. When the surface tension is constant, the fluid surface is like a taut rubber sheet. Poke a hole in that sheet, and everything pulls away from the hole. Likewise, when the surface tension varies, fluid will move from areas of low surface tension toward areas of higher surface tension. This effect is easily demonstrated at home in a setup like the animation above. Pour milk (higher fat content is better) and food coloring in a shallow container. Then lower the local surface tension using dish soap or rubbing alcohol and watch the colors run away! (Image credit: Flow Visualization at UC Boulder, source video)

Healing Bubbles
Soap bubbles are ephemeral creations. The slightest prick will send them tearing apart in the blink of an eye. It may come as a surprise, therefore, that dropping a water droplet through a bubble will not break it. Instead, the bubble will heal itself using the Marangoni effect. In a soap bubble, the soap molecules act as a surfactant, lowering the surface tension of the water and allowing the fragile structure to hold together. When the water drop impacts the bubble, the local surface tension increases because of the relative lack of soap molecules. This increase in surface tension pulls at the rest of the bubble, drawing more soap molecules toward the point of contact. The effect evens out surface tension across the surface and stabilizes the bubble. You can test the effect at home, too. If you wet your finger, you can poke a soap bubble without popping it. (Video credit: G. Mitchell; via io9)

Distorted Rings
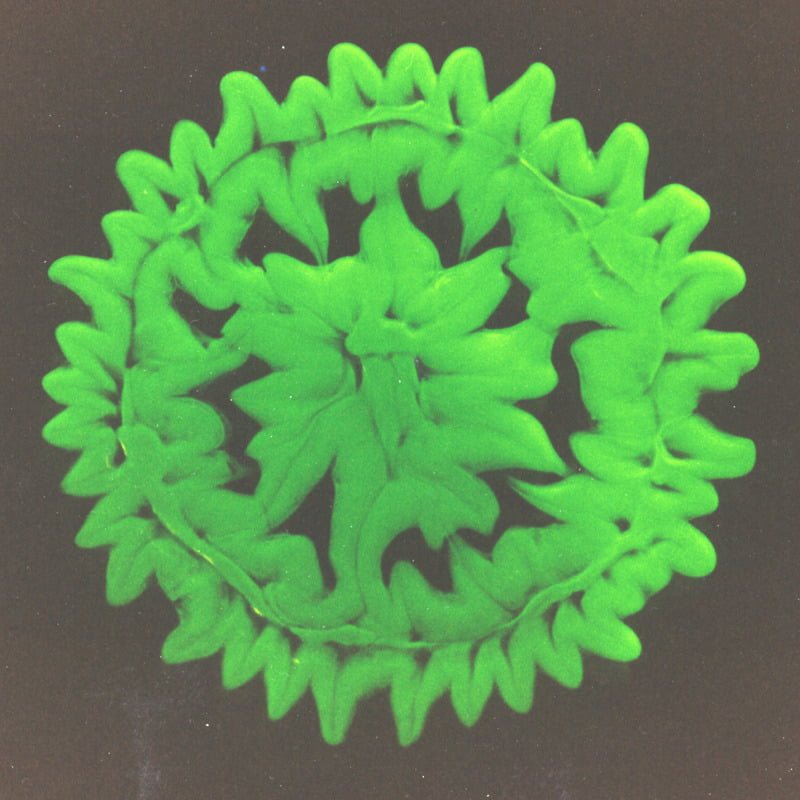
The Marangoni effect is generated by variations in surface tension at an interface. Such variations can be temperature-driven, concentration-driven, or simply due to the mixing between fluids of differing surface tensions as is the case here. The pattern in the image above formed after a dyed water droplet impacted a layer of glycerin. The initial impact of the drop formed an inner circle and outer ring. This image is from 30 seconds or so after impact, after the Marangoni instability has taken over. The higher surface tension of the water pulls the glycerin toward it, resulting in a flower-like pattern. (Photo credit: E. Tan and S. Thoroddsen)

Inksplosion
Chemical Bouillon are a trio of artists who use the chemistry of surface reactions to create abstract videos full of exploding and imploding droplets and colors. As chemicals react, local concentrations at the interface vary, which changes the local surface tension. These gradients drive flow from areas of low surface tension to those of higher surface tension. This is called the Marangoni effect – the same behavior that drives tears in a glass of wine. Chemical Bouillon have a whole YouTube channel dedicated to these kinds of videos, with everything from inks to ferrofluids. Be sure to take a look at some of their other videos and, if you like them, subscribe. (Video credit: Chemical Bouillon)

Holey Splashes
A liquid’s surface tension can have a big effect on its splashes. In this video, a 5-mm droplet hits a surface covered in a thin layer of a liquid with lower viscosity and surface tension. The result is a dramatic effect on the spreading splash. As the initial curtain grows and expands, the lower surface tension of the impacted fluid thins the splash curtain. Fluid flows away from these areas due to the Marangoni effect, causing holes to grow. The sheet breaks up into a network of liquid filaments and ejected droplets before gravity can even bring it all to rest. For more, see this previous post and review paper. (Video credit: S. Thoroddsen et al.)

Marangoni Flows
Differences in surface tension cause fluid motion through the Marangoni effect. Because an area with higher surface tension pulls more strongly on nearby liquid than an area of low surface tension, fluid will flow toward areas of higher surface tension. Here surfactants, shown in white, are constantly injected onto a layer of water dyed blue. You can also see the flow in motion in this video. Outside of the central source flow, the pattern features lots of 2D mushroom-like shapes reminiscent of Rayleigh-Taylor instabilities. But these shapes are driven by variations in surface tension rather than unstable density variations. For more, check out the original paper or learn about other examples of Marangoni effect. (Photo credit: M. Roché et al.)