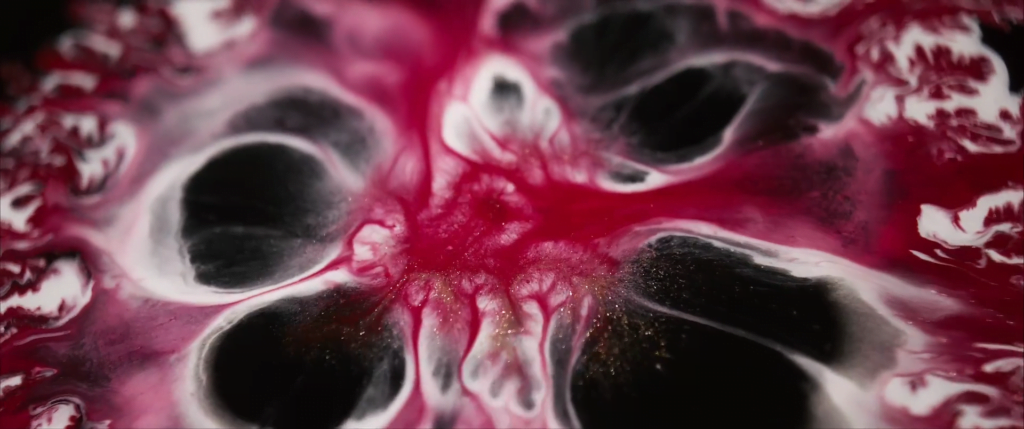
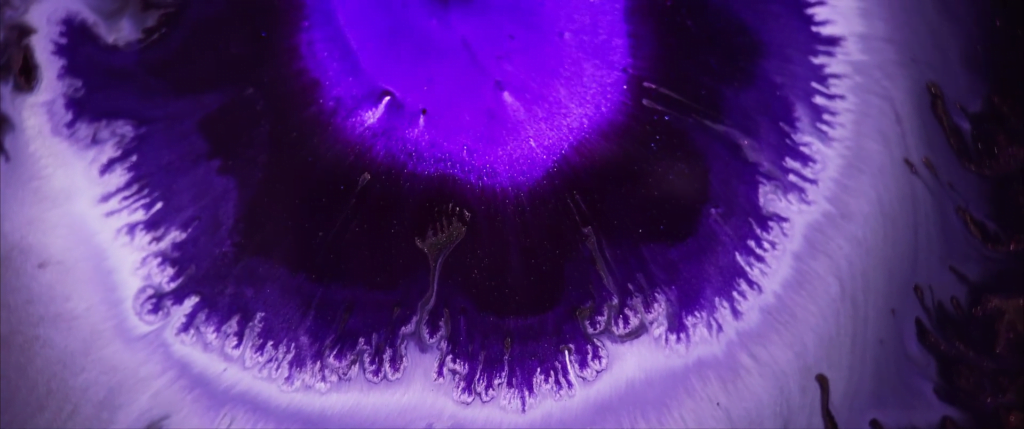
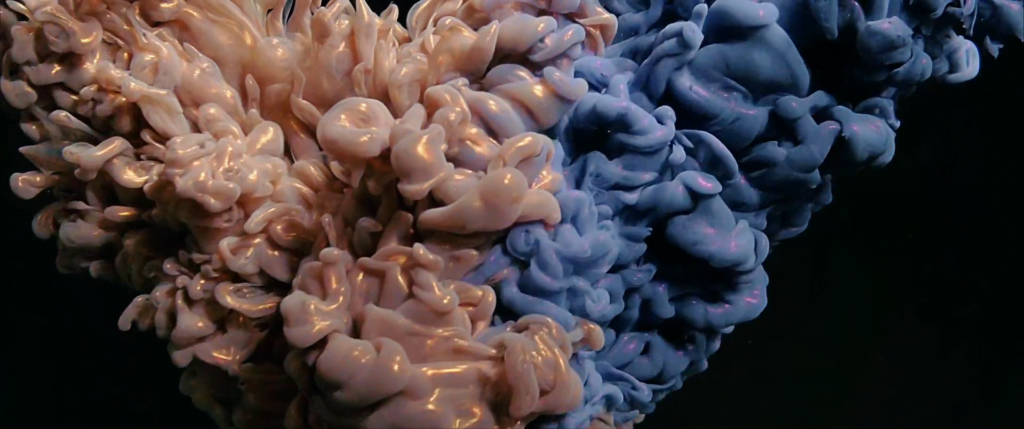
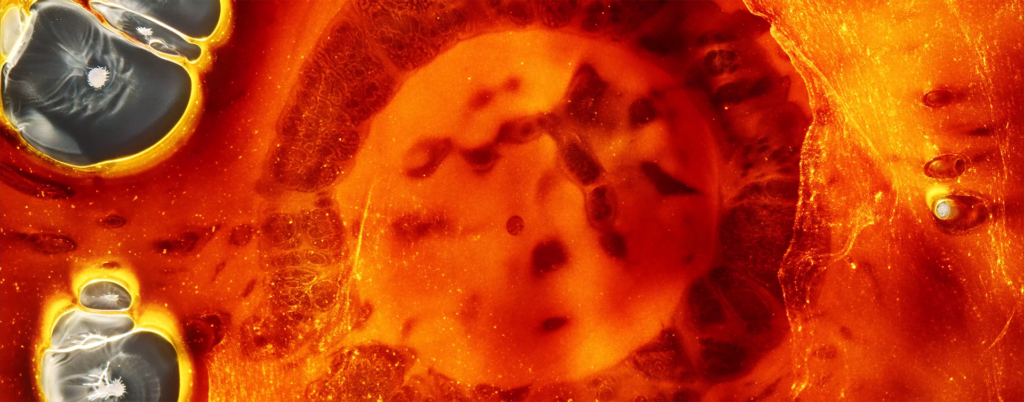
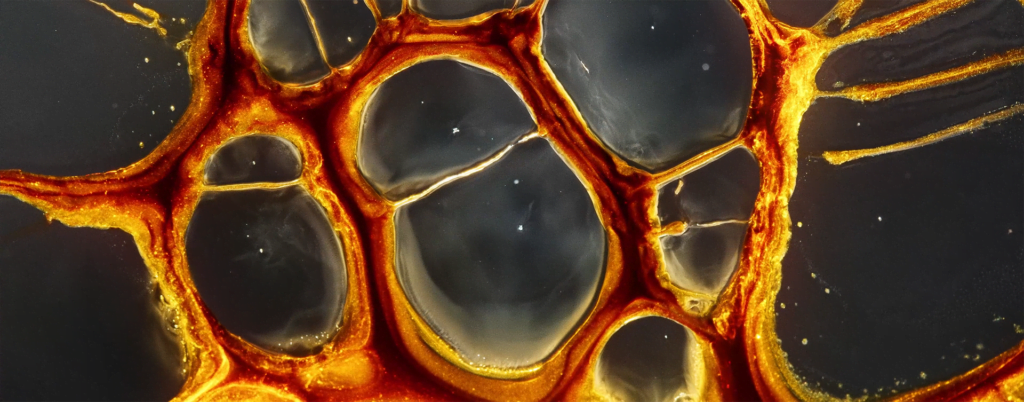
Billowing turbulence, mushroom-like Rayleigh-Taylor instabilities, and spreading flows abound in Vadim Sherbakov’s “Origin.” The short film takes a macro looks at fluids — inks, alcohols, soaps, and other household liquids. It was filmed entirely on a DJI Pocket 2, a rather small, stabilized pocket camera. It’s a testament to what you can achieve with some experimentation and relatively inexpensive equipment. (Video and image credit: V. Sherbakov)
Tag: surface tension

“Lucid”
Artist Roman Hill made this official music video to go with Thomas Vanz’s “Lucid.” The imagery, formed from ink and other fluids, warps our sense of scale. Though the camera focuses on an extremely small area, to our eyes the results shift from nebulas to oceans and back again. There are likely a whole host of phenomena going on here, but without knowing more about Hill’s ingredients, I can only speculate that there are Marangoni flows driven by variations in surface tension and maybe some density instabilities going on between fluid layers. I’m also fairly confident that Hill has played with time reversal in the video editing. Regardless of the secrets in its making, the film is captivating and gorgeous. (Image and video credit: R. Hill)

“Perfect Sky”
It’s all blue skies in Roman De Giuli’s short film, “Perfect Sky.” Created with paint, ink, and glitter on paper, it’s a relaxing piece of fluid art. Put on your headphones, take a deep breath, and plunge in. You’ll see lots of gorgeous Marangoni effects, some low Reynolds number mixing, and various instabilities. (Video and image credit: R. De Giuli)

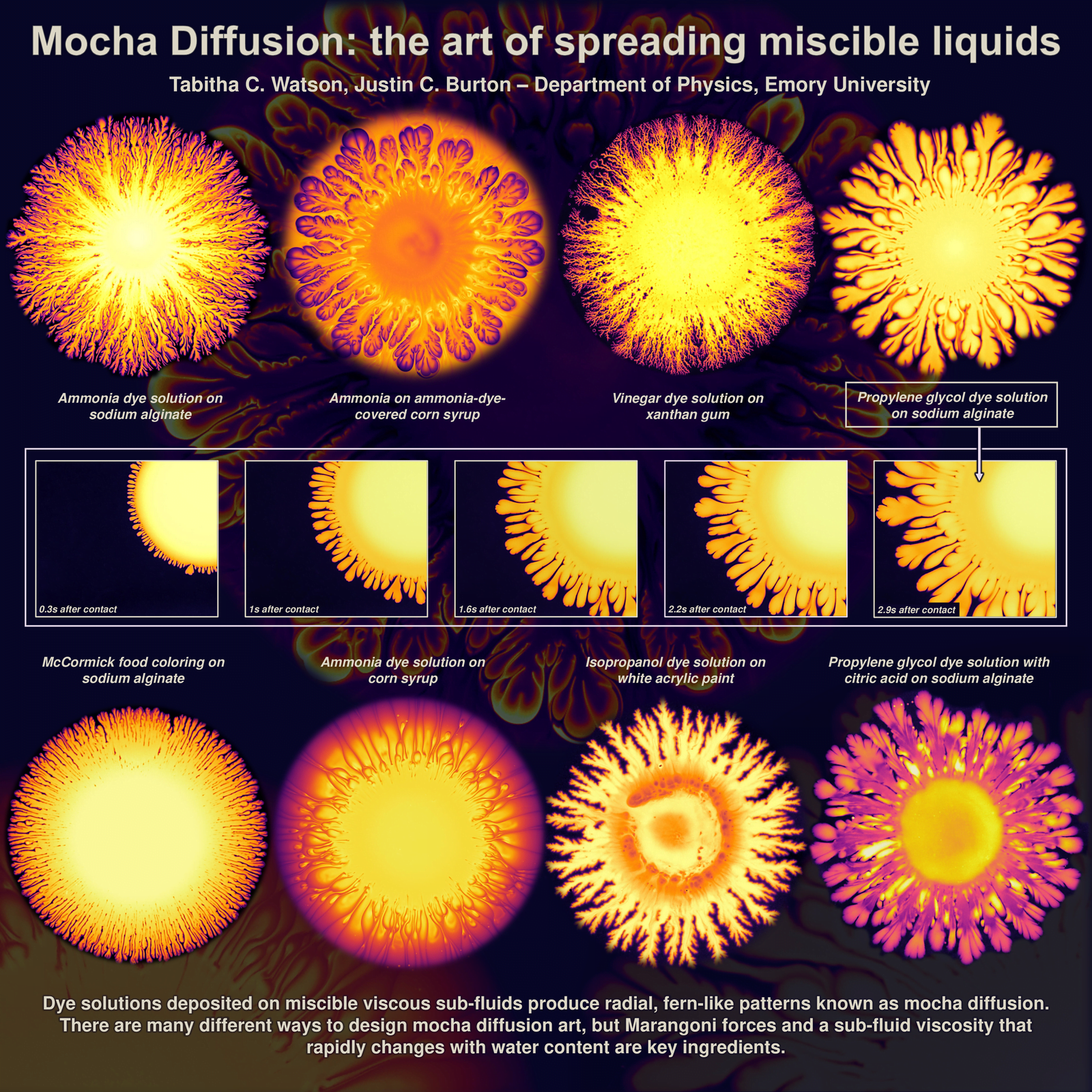
Mocha Diffusion
These firework-like patterns spread when dyes are added atop a viscous but miscible lower fluid layer. Here, researchers use lower layers like corn syrup and xanthan gum; then they spread dye mixtures including ammonia and vinegar atop those layers. Because the upper and lower layers of fluid are miscible and can diffuse into one another, they together form elaborate patterns. The mixing of the two layers creates gradients in surface tension that can drive the flow and create these mocha diffusion patterns. (Image credit: T. Watson and J. Burton)

“High Flow”
Roman De Giuli’s “High Flow” is vibrant and energetic. Colorful paints and inks flow across the page, creating complex patterns. I love the blossoming flows, feathery fronds, and spreading Marangoni effects. De Giuli’s films never disappoint! (Video and image credit: R. De Giuli)

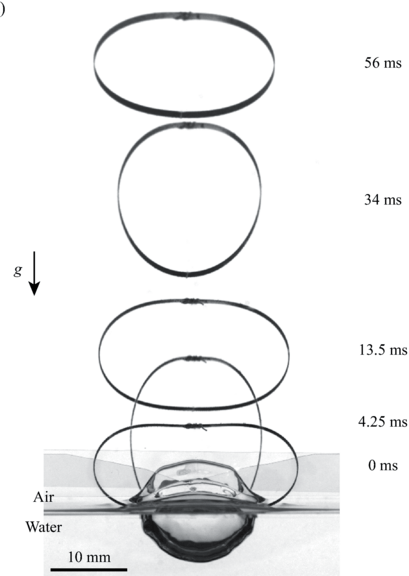
Water Jumping Hoops
Small creatures like springtails and spiders can jump off the air-water interface using surface tension. But larger creatures can water-jump, too, using drag. Here, researchers study drag-based water jumping with a simple elastic hoop. Initially, two sides of the hoop are pulled closer by a string, deforming the hoop. Then, with the hoop sitting upright on the air-water interface, a laser burns the string, releasing the energy stored in the hoop. The hoop’s bottom pushes into the water, generating drag. That resistance provides a reaction force strong enough to launch the hoop.
Compared to the hoop’s jumps off land, it’s slower to take-off from water, and it’s less efficient at jumping. Lighter hoops, however, jump better off water than heavier ones — a wrinkle that isn’t seen in ground jumpers. That suggests that weight reduction is more important for aquatic jumpers than for their terrestrial counterparts. (Image and research credit: H. Jeong et al.)

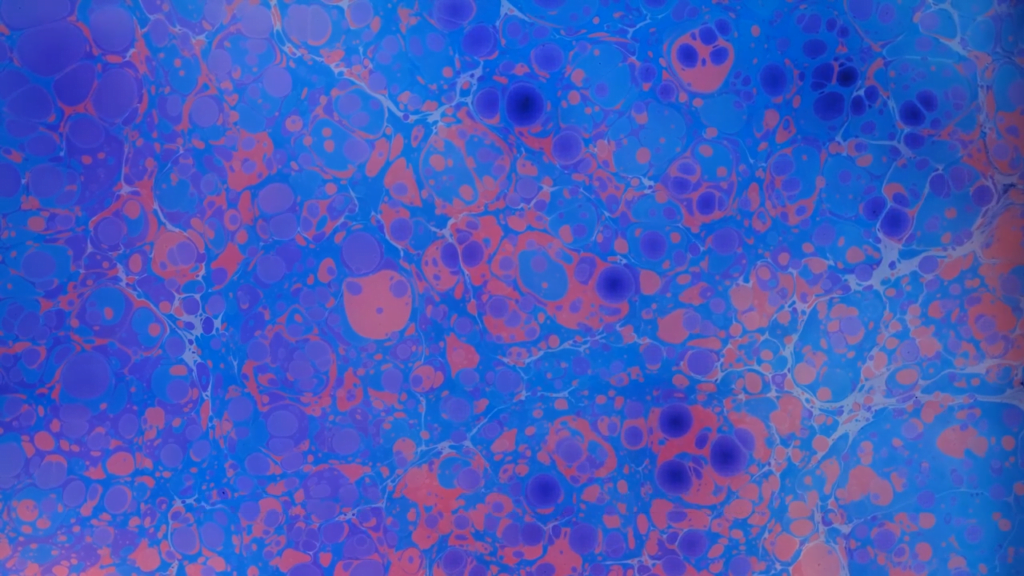
The Hydrodynamics of Marbling
In marbling, an artist floats paints on a viscosified water bath, using various thin tools to manipulate the final image. Many cultures have developed a version of this art, but for many it will be most recognizable as a technique used to decorate book interiors. In this video, researchers consider the physics behind this beautiful practice. Surface tension helps keep the paint on the surface, even though it’s denser than the water it’s on. Variations in surface tension shape and reshape the surface as new colors are added. And then low-Reynolds-number effects help artists mix the paints without inertia or diffusion disturbing the pattern. See more examples here, here, and here. (Video credit: Y. Sun et al.)

Dancing to Chopin
Droplets of paint whirl to Chopin’s “Nocturne Op. 9 No. 2” in this short film from artist Thomas Blanchard. The glitter particles in the paints act as seed particles that highlight the flow within and around each drop. It’s a beautiful dance of surface tension, advection, and buoyancy. (Image and video credits: T. Blanchard; via Colossal)

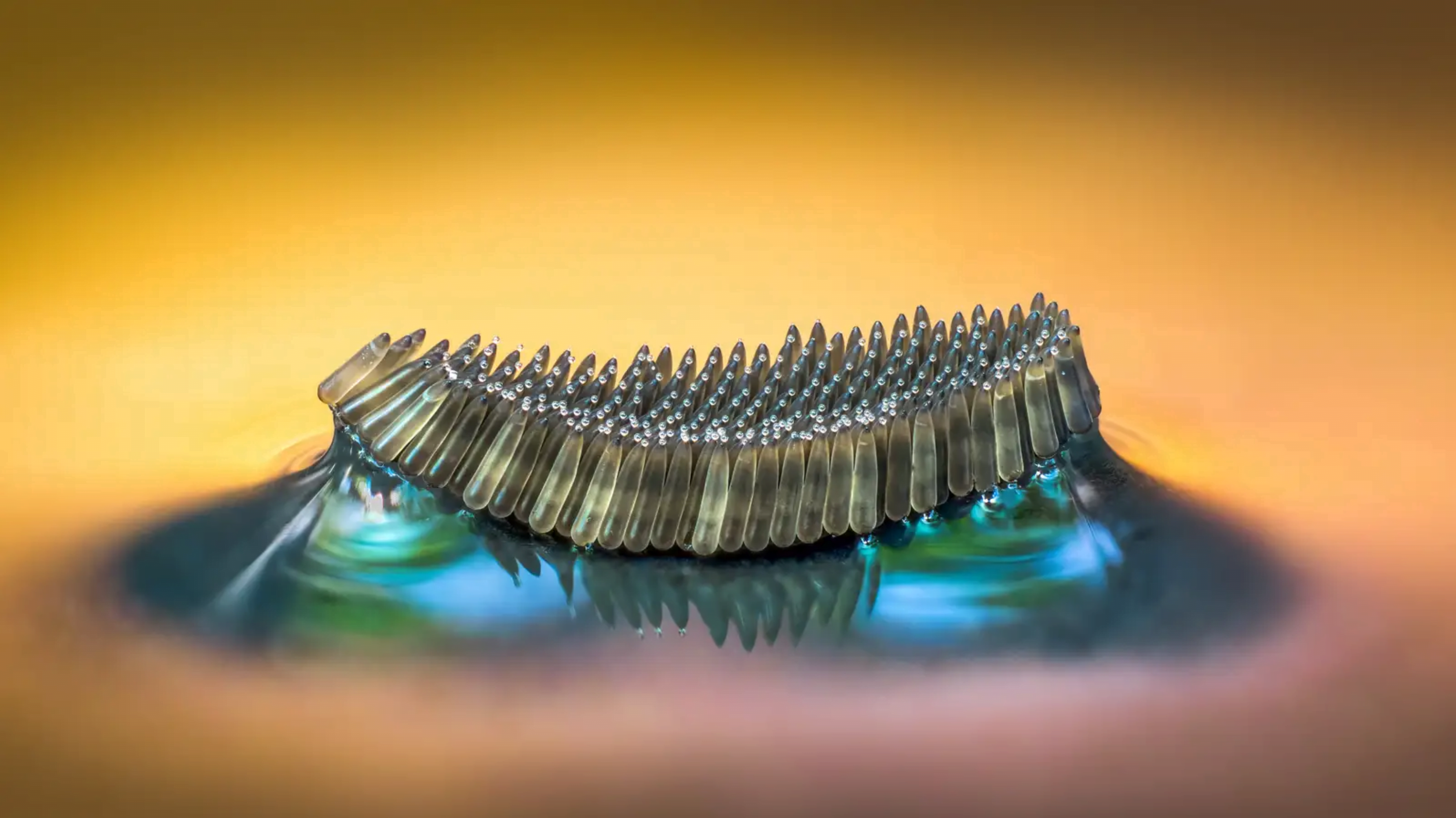
Enhancing the Cheerios Effect
The Cheerios in your morning cereal clump together with one another and the bowl’s wall due to an attractive force caused by the curvature of their menisci. A recent study looks at how this effect changes when you’re pulling objects out of the liquid.

Snapshots show how two flexible fibers get drawn together by an attractive force as they are pulled out of silicon oil. The researchers inserted thin flexible glass fibers into silicon oil and withdrew them. As they did, they explored what lengths and retraction speeds caused the fibers to pull together. They found that a single moving rod had a taller meniscus than a stationary one, and two moving rods had a liquid bridge that superposed their individual menisci. The result was an attractive force even stronger than what the fibers experienced when still. (Image credit: Cheerios – D. Streit, experiment – H. Bense et al.; research credit: H. Bense et al.; via APS Physics)