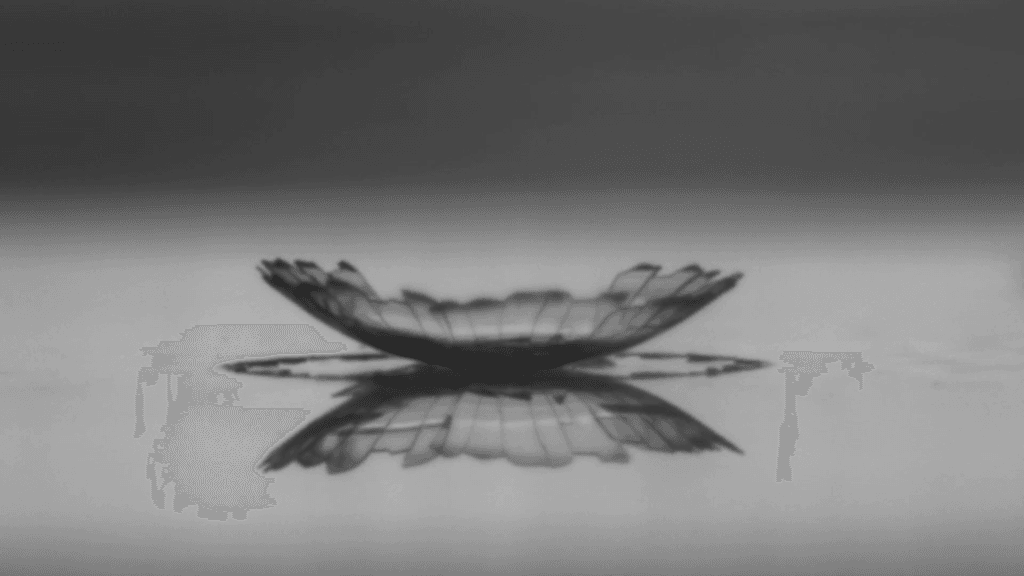
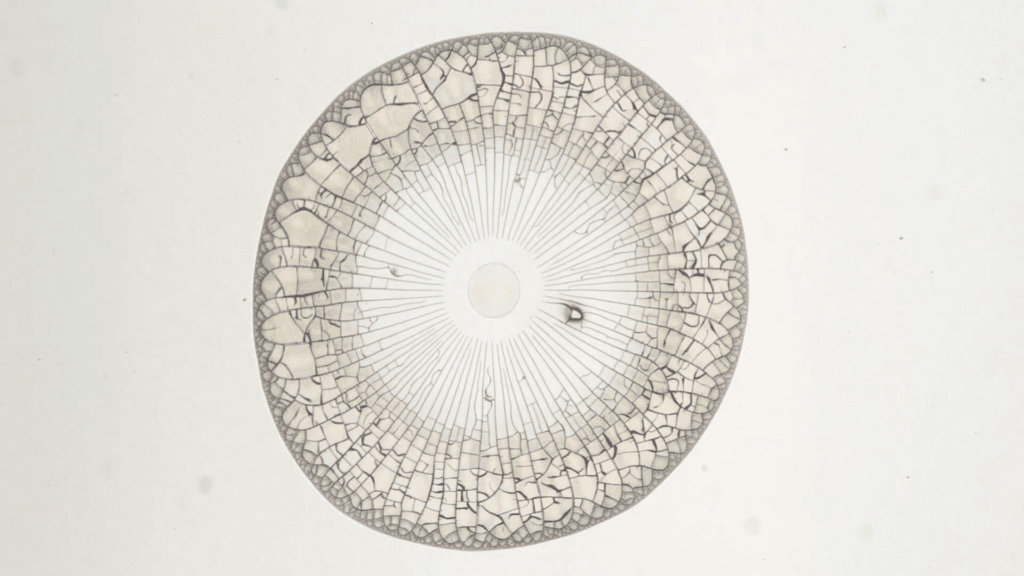
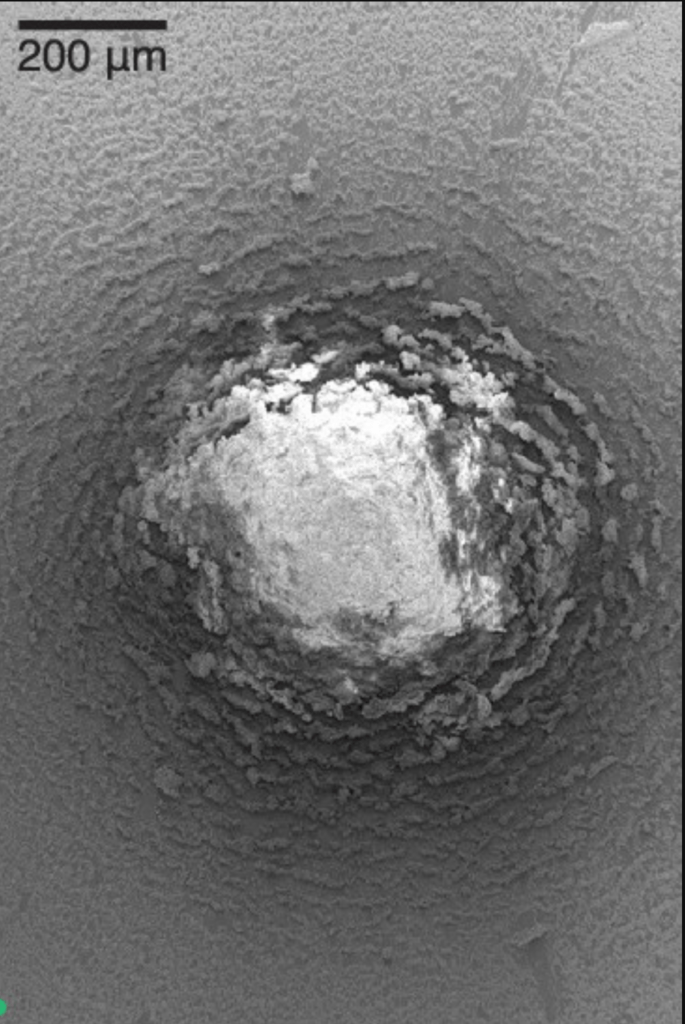
When millimeter-sized drops of water infused with nanoparticles dry, they leave behind complex and beautiful residues. As water continues evaporating, the residues warp, bend, and crack. In this video, researchers set their science to the music of Leonard Cohen. The results resemble blooming flowers and flying water fowl. If you’d like to learn more about the science behind the art, check out the two open-access papers linked below. (Video and image credit: P. Lilin and I. Bischofberger; submitted by Irmgard B.; see also P. Lilin and I. Bischofberger and P. Lilin et al.)
Tag: coffee rings

Freezing Stains
When they evaporate, drops of liquids like coffee and red wine leave behind stains with a darker ring along the edges, thanks to capillary action and surface tension pulling particles to that outer edge. In contrast, sublimating a frozen droplet leaves a stain pattern that concentrates at the center (top). When droplets freeze from the surface upward, particles within the droplet are driven toward the center as the freeze front pushes toward the drop apex. The final shape of the stain depends on the initial geometry of the droplet, and the concentration of particles toward the center occurs because of the way that the particle freezes, not how it sublimates (bottom).
Since many industrial processes rely on droplet evaporation to spread coatings, this work offers a new way to control the final outcome. (Image and research credit: E. Jambon-Puillet, source)

How Coffee Rings Form
Coffee rings (an ubiquitous feature of academia) are formed by the deposition of particles as the liquid evaporates. When a coffee drop evaporates, capillary action draws the coffee particles toward the edges of the drop, where they congregate into a ring. Research now suggests that this is due to the spherical nature of the particles. Ellipsoidal particles, in contrast, clump together and result in a uniform stain once their carrier liquid evaporates. The effect seems to be due to the particles’ effects on surface tension; the ellipsoidal particles deform the surface of the droplet as it evaporates such that they are not pulled to the edges. Adding a surfactant, like soap, that decreases surface tension caused the ellipsoidal particles to form rings just as the spherical particles do. (submitted by Neil K) #