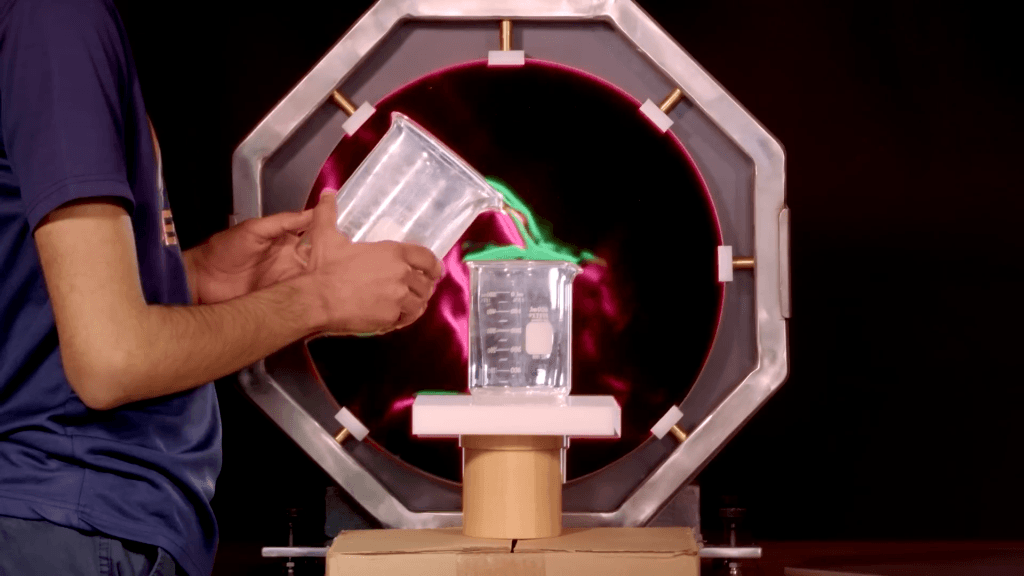
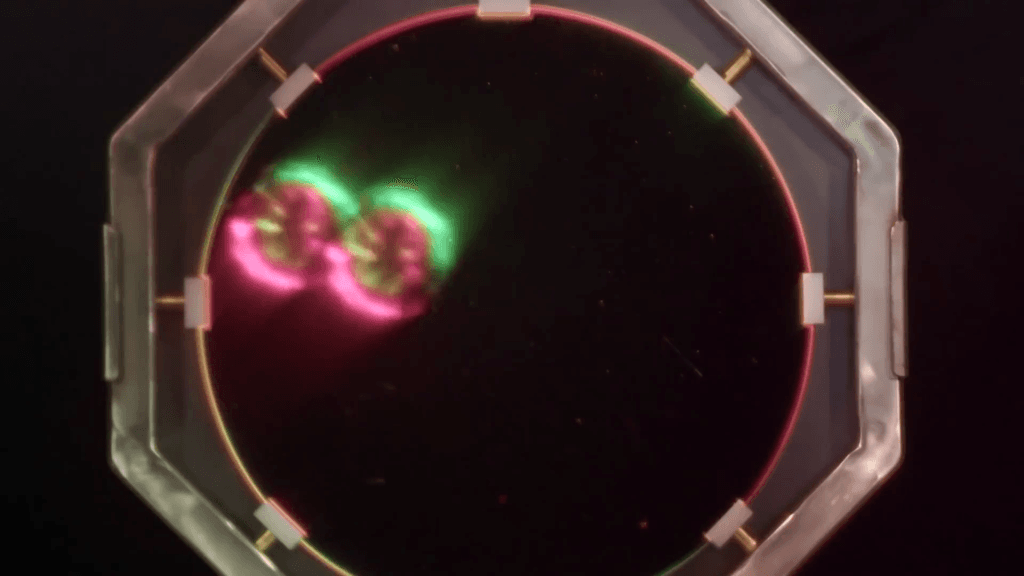
When hurricanes hit coasts, they bring with them incredible storm surge, which puts buildings right in the middle of ocean waves. To understand how to better protect against those conditions, engineers use facilities like the Directional Wave Basin to create smaller-scale versions of hurricanes. In this Practical Engineering video, Grady visited during a test that compared two identical one-third-scale houses subjected to the same storm conditions–except that one house had an additional foot (3ft at real-scale) of elevation. The results are pretty spectacular.
This isn’t a short video, but it’s well-worth a watch. I think Grady does a great job of explaining why engineers need (admittedly) expensive facilities like this one to help guide both engineering and regulatory decisions. (Video and image credit: Practical Engineering)