Two liquids that collide don’t always coalesce. The image above shows two jets of silicone oil colliding. On the left, the jets collide and bounce off one another. On the right, at a slightly higher flow rate, the two jets coalesce. This bouncing, or noncoalescence, observed at lower speeds is due to an incredibly thin layer of air separating the two jets. This air layer is constantly being replenished by air that gets dragged along by the flowing oil. But if the oil flows too quickly, that air layer becomes unstable–in the same way that a droplet that falls too quickly will splash on impact. When the separating air layer becomes unstable and breaks down, the jets collide and merge. (Image credit: N. Wadhwa et al., pdf)
Tag: lubrication

Living Fluid Dynamics
This short film for the 2016 Gallery of Fluid Motion features Montana State University students experiencing fluid dynamics in the classroom and in their daily lives. As in her previous film (which we deconstructed), Shanon Reckinger aims to illustrate some of our everyday interactions with fluids. This time identifying individual phenomena is left as an exercise for the viewer, but there are hints hidden in the classroom scenes. How many can you catch? I’ve labeled some of the ones I noticed in the tags. (Video credit: S. Reckinger et al.)

Why Joints Pop
Joints like our knuckles are lubricated with liquid called the synovial fluid. When manipulated, these joints can pop or crack audibly. For half a century, researchers have thought the cracking sound joints under tension make was the result of bubbles in the synovial fluid collapsing. But a new cine magnetic resonance imaging (MRI) study shows that the sound is generated during bubble inception and that the cavity persists after the sound. When the bones of the joint are pulled, viscous forces resist their separation. With enough force, the joints separate suddenly, causing a pressure drop in the synovial fluid that forms a vapor-filled cavity in the joint. According to the real-time MRI observations, this is when the sound is generated. The cavity does eventually dissipate, they found, but only well after the pop. The whole joint-cracking process is consistent with the tribonucleation mechanism seen in machinery. (Image credit: G. Kawchuk et al.; GIF via skunkbear, source video)

Levitating Droplets with Motion
There are many ways to levitate a droplet – heating, vibration, and acoustic levitation all come to mind – but this video demonstrates a simpler method: a moving wall. Depositing a drop on a moving wall keeps it aloft with a thin, constantly replenished layer of air. The thickness of this lubricating air film is directly measurable from interference fringes created by light reflecting off the surface of the drop. Incredibly, the air layer is only a few microns thick, but the resulting pressure in the air film is high enough to levitate millimeter-sized droplets! (Video credit: M. Saito et al.; via @AlvaroGuM)

Lakes Upon Glaciers
Supraglacial lakes–ephemeral bodies of water that form atop glaciers–can form and empty in a matter of hours. The lakes typically empty either by overflowing their banks or by discharging through a moulin, a well-like crevasse in the ice. When this happens, the water from the lake drains into the bed beneath the glacier, acting like a lubricant between the ice and the land and thus accelerating the glacier’s movement. The team in the video studied the draining of two different lakes, one which voided within 2 hours and the other slower one which drained over 45 hours. The faster of the two accelerated the glacier’s movement to a maximum of 1600 meters/year, far higher than its baseline velocity of 90-100 meters/year. For more see Laboratory Equipment and this post on ice flow. (Video credit: City College of New York)

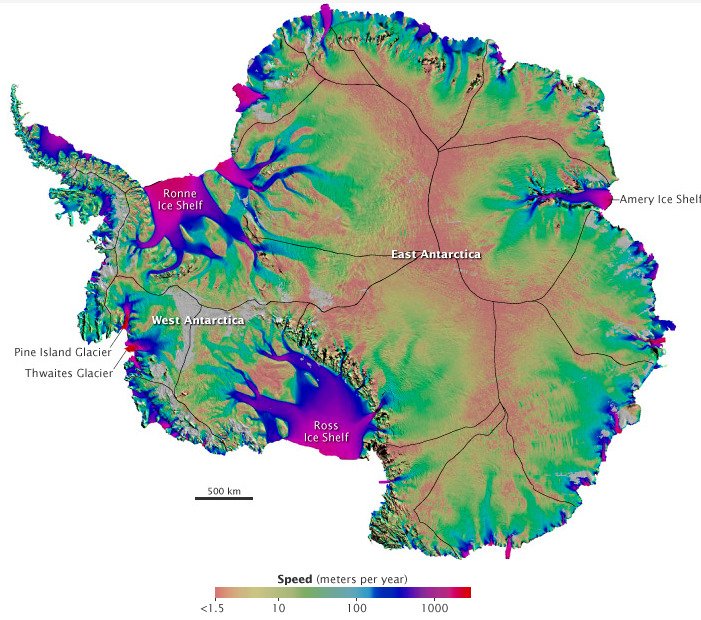
Antarctic Ice Flows

Even frozen ice moves and flows, though too slowly to see with the naked eye. By combining satellite imagery from NASA, JAXA, CSA, and ESA, researchers were able to map the flow of ice across Antarctica, discovering ice streams (shown in blue and purple above) that can move hundreds of meters a year. The dynamics of this motion are still poorly understood, with theoretical advances underway. These ice sheets sit atop bedrock that is itself below sea level. A thin layer of water exists between the ice sheet and the bedrock, acting as a lubricant and allowing the ice to slide against the bedrock. To see animations of Antarctic ice flow, see this compilation film. (Photo credits: E. Rignot/NASA JPL/UC Irvine #; M. J. Hambrey #)

Getting Ketchup to Flow
Most everyone is familiar with the difficulty of getting ketchup out of its bottle. Part of the trouble is that ketchup is a shear-thinning fluid, meaning that its viscosity decreases with an increasing rate of shear. Thus, a shear-thinning fluid flows better once it starts moving. This is why the ketchup moves much faster once it is initially disturbed. LiquiGlide, a new coating material demonstrated above, has gained a lot of popular attention in the press recently for solving the difficulty of the stuck condiments. It appears that the coating reduces the static coefficient of friction between the food and the bottle, meaning that the ketchup starts sliding down the wall even before an increase in shear stress starts the flow. (submitted by @szescstopni)

Liquid Nitrogen and the Leidenfrost Effect
One of the tried and true cooking tips my mother gave me when I was younger was to test the temperature of my griddle before making pancakes by splashing a few drops of water on it. If it was hot enough that the water skittered across the surface before evaporating, then it was ready. Aside from being a way to make great pancakes, this tip demonstrates an everyday application of the Leidenfrost effect. When the surface of the pan is significantly higher than the boiling point of the water, the part of the water drop that hits the pan is vaporized, creating a thin layer of water vapor on which the rest of the droplet rests. The vapor serves as an insulator, protecting the rest of the water drop from the heat of the pan, as well as a lubricant, allowing the drop slip and slide easily across the surface. The same effect lets the brave plunge a hand into liquid nitrogen without damage, but they have to be quick, otherwise their hand will cool to the point that the liquid nitrogen contacts it without a protective layer of nitrogen. (In that case, a nasty case of frostbite may be the least of one’s worries. We do NOT recommend trying this one at home.)

Wave-Particle Duality in Bouncing Droplets
A droplet atop a vibrating pool is prevented from coalescing by the constant influx of air into a thin lubrication layer between it and the pool. But that is not the strangest aspect of its behavior. Researchers have found that this system demonstrates some aspects of the mind-bending wave-particle duality at the heart of quantum physics. (Submitted by Dan H.) #

Hot Spheres Sink Faster
New research shows that the Leidenfrost effect–which causes water droplets to skitter across a hot pan–can drastically reduce the drag on objects moving through a liquid. When raised to a high enough temperature, a sphere falling water will be coated in a protective layer of vapor (see video above) that acts like a lubricant as the sphere moves through the water. If the temperature of the object drops too low, the vapor layer will dissolve into a mess of bubbles (~35 secs into video). One way that this mechanism reduces drag is by keeping flow attached to the sphere for longer as shown in this video. Preventing this flow separation increases the pressure recovered after the point of lowest pressure (the shoulders of the sphere), which reduces overall drag.
See also:
- PRL Article and Supplemental Materials
- Wired article
- The Photonist