Engineering classes often discuss hydrostatics–the physics of non-moving water–before they cover fluid dynamics and its flows. But hydrostatics is plenty challenging on its own, as Steve Mould demonstrates in this video looking at how hydrostatic pressure depends on depth (and, not, as our intuition might suggest, on shape). As always, he has some nice countertop-scale demos to go with it. (Video and image credit: S. Mould)
Tag: DIY fluids

Competing Time Scales
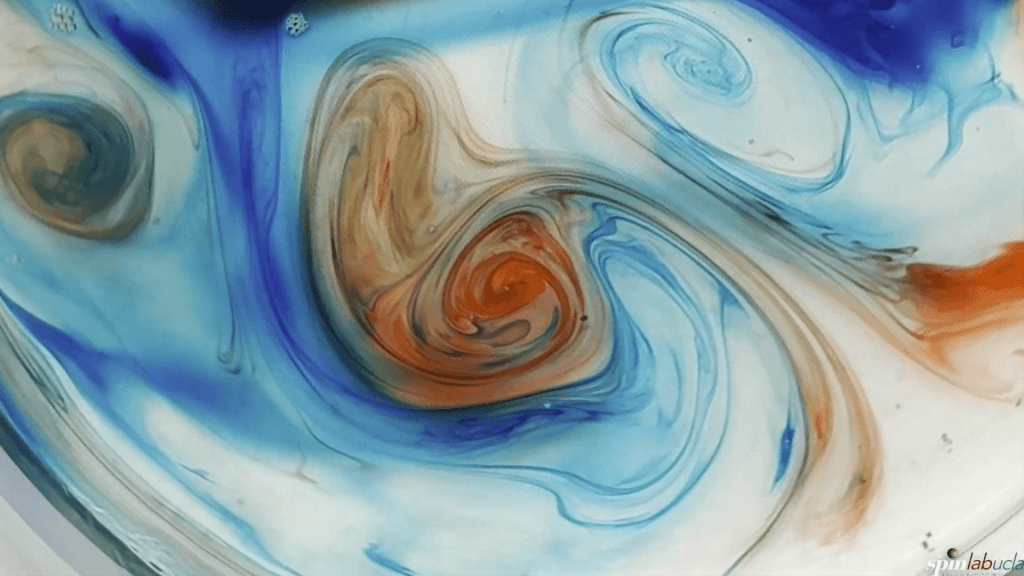
Fluid dynamics often comes down to a competition between the different forces acting in a flow. Inertia, surface tension, viscosity, gravity, rotation — flows can be affected by all of these and more. In this video, researchers describe the three dominant forces in a rotating fluid like a planet’s atmosphere: viscosity, the fluid’s resistance to flowing; inertia, the fluid’s resistance to accelerating; and rotation, the overall spin of a fluid.
As shown in the video, which of these three forces dominates will change depending on the speed at which the force acts. We quantify this concept using time scales; the force with the smallest time scale can act fastest and will, therefore, win the tug-of-war. (Video and image credit: UCLA SpinLab)

Liquefaction in Earthquakes
In an earthquake, sand and soil particles get jostled together, forcing any water between them up toward the surface. The result is liquefaction, a state where once-solid ground starts to behave much like a liquid. Buildings can tip over and pipelines get pushed toward the surface. In this video, a geologist shows off some great demonstrations of the effect, including ones that can be easily done in a classroom with younger kids. (Video credit: California Geological Survey)

What Limits a Siphon
Siphons are a bit mind-boggling for anyone who has internalized the idea that water always flows downhill. But gravity actually allows a siphon’s water to flow up and over an obstacle, provided certain conditions are met. Steve Mould digs into the details of those conditions in this video, where he searches for the maximum height a siphon can reach.
A quick note on terminology: Steve explains that the siphon breaks when water near the top starts “boiling.” Other sources may use the term “cavitating” for this sudden phase change. There’s not–to my knowledge–a generally-agreed-upon definition that clearly distinguishes between boiling and cavitation in this situation. Whichever term you use, the water in the siphon doesn’t care; either way, it’s experiencing a local pressure that’s so low that it switches from a liquid state (where it can resist tensile forces) to a gaseous one (where it cannot resist tension). (Video and image credit: S. Mould)

Spinning Water
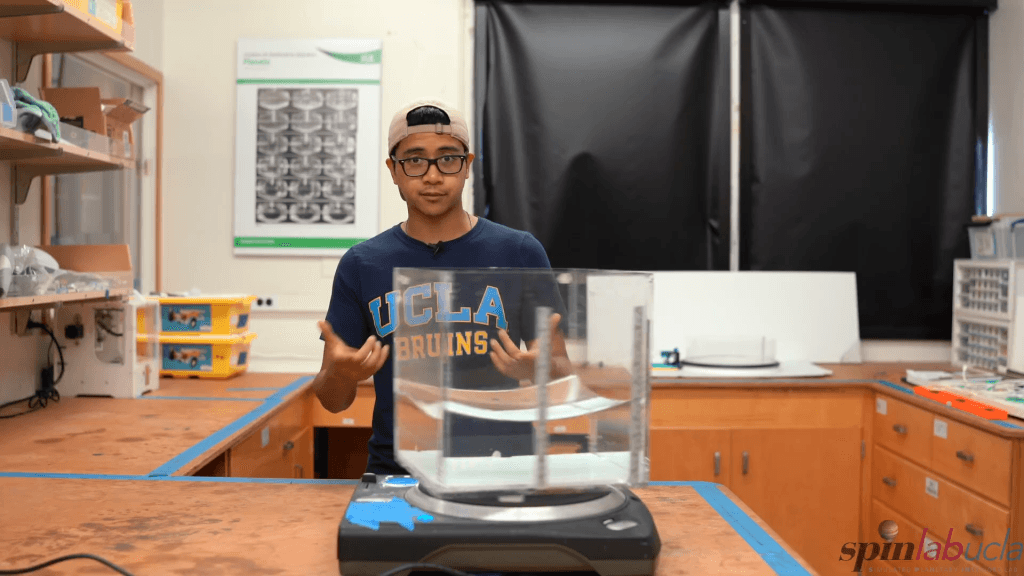
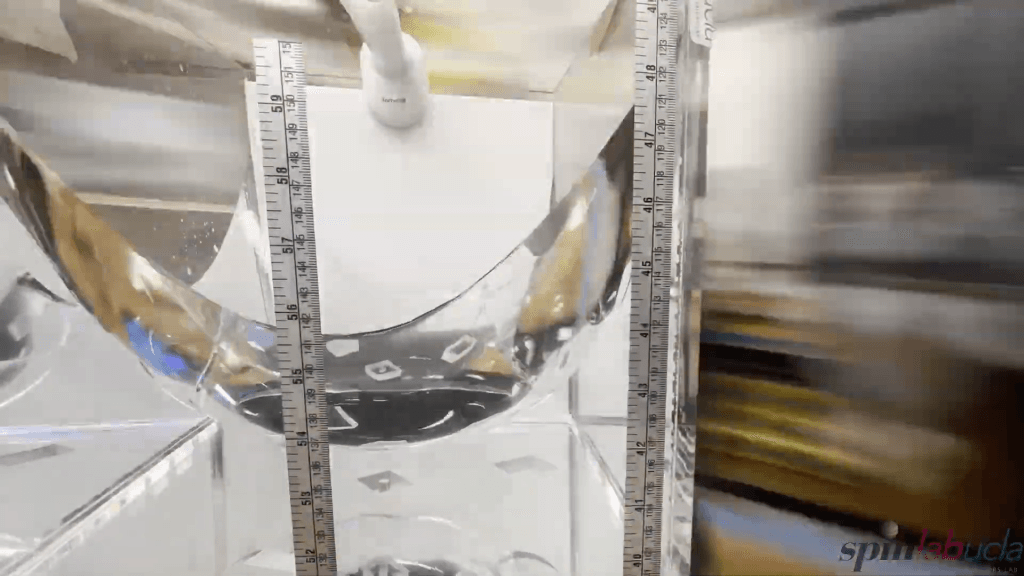
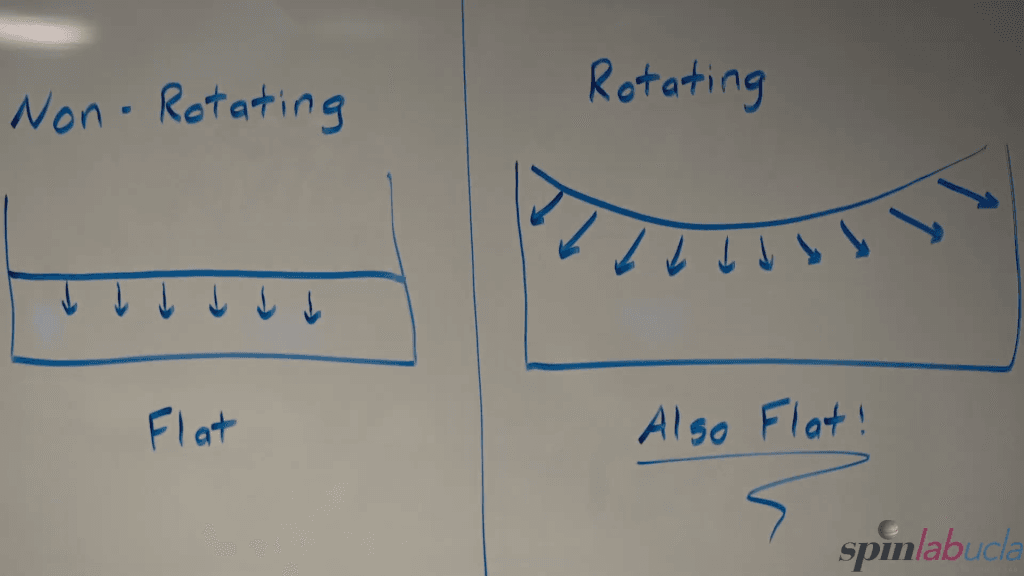
If you spin a tank of water at a constant speed, it takes on a curved, parabolic shape–a demonstration often called Newton’s bucket. Here, a team from UCLA shows how it’s done, both in terms of the equipment needed and a concise explanation of the physics. In the rotating experiment, water is subjected to both gravity (which acts in a constant magnitude across the tank) and centrifugal force (which is stronger further from the axis of rotation). The shape that balances these forces is a paraboloid, which is why the water takes on that shape. (Video and image credit: UCLA SpinLab)

Active Cheerios Self-Propel
The interface where air and water meet is a special world of surface-tension-mediated interactions. Cereal lovers are well-aware of the Cheerios effect, where lightweight O’s tend to attract one another, courtesy of their matching menisci. And those who have played with soap boats know that a gradient in surface tension causes flow. Today’s pre-print study combines these two effects to create self-propelling particle assemblies.
The team 3D-printed particles that are a couple centimeters across and resemble a cone stuck atop a hockey puck. The lower disk area is hollow, trapping air to make the particle buoyant. The cone serves as a fuel tank, which the researchers filled with ethanol (and, in some cases, some fluorescent dye to visualize the flow). Like soap, ethanol’s lower surface tension disrupts the water’s interface and triggers a flow that pulls the particle toward areas with higher surface tension. But, unlike soap, ethanol evaporates, effectively restoring the interface’s higher surface tension over time.
With multiple self-propelling particles on the interface, the researchers observed a rich series of interactions. Without their fuel, the Cheerios effect attracted particles to each other. But with ethanol slowly leaking out their sides, the particles repelled each other. As the ethanol ran out and evaporated, the particles would again attract. By tweaking the number and position of fuel outlets on a particle, the researchers found they could tune the particles’ attractions and motility. In addition to helping robots move and organize, their findings also make for a fun educational project. There’s a lot of room for students to play with different 3D-printed designs and fuel concentrations to make their own self-propelled particles. (Research and image credit: J. Wilt et al.; via Ars Technica)

“Origin”
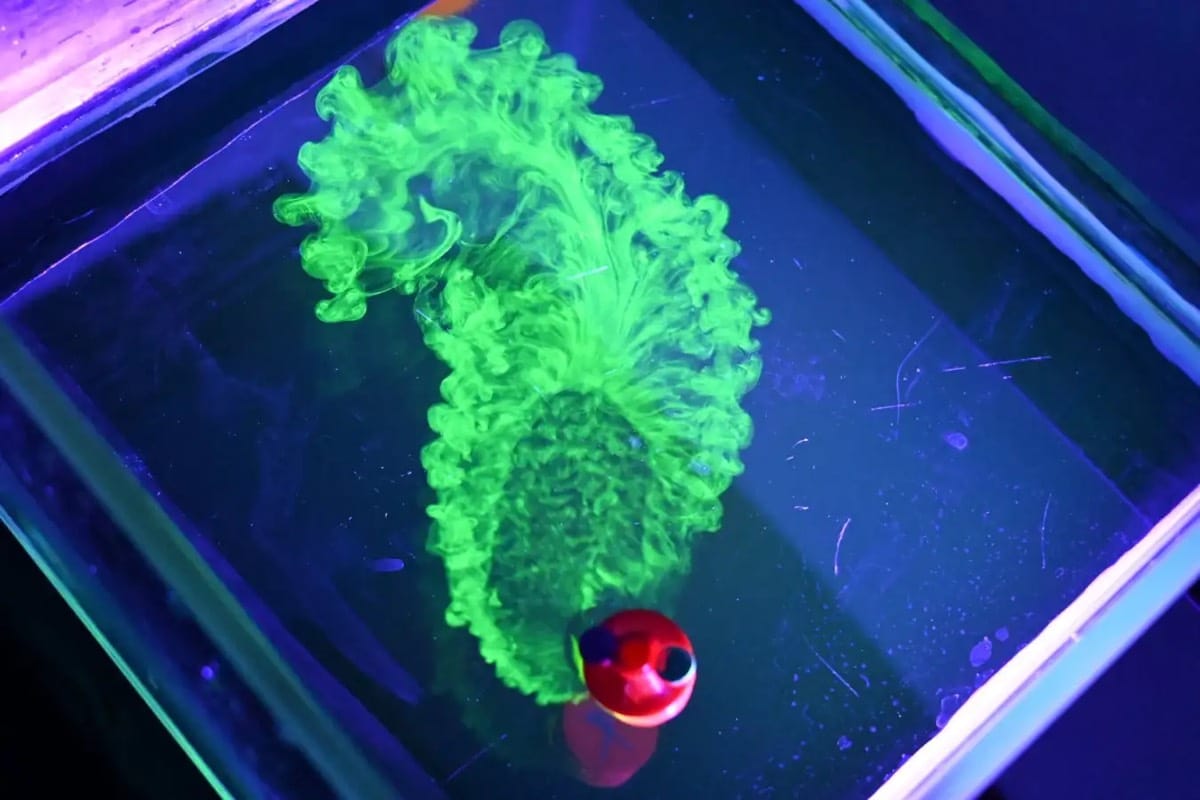
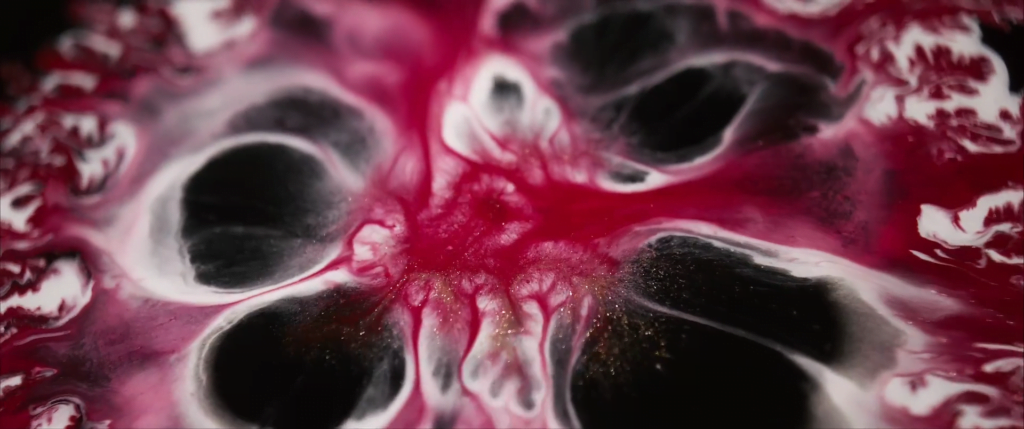
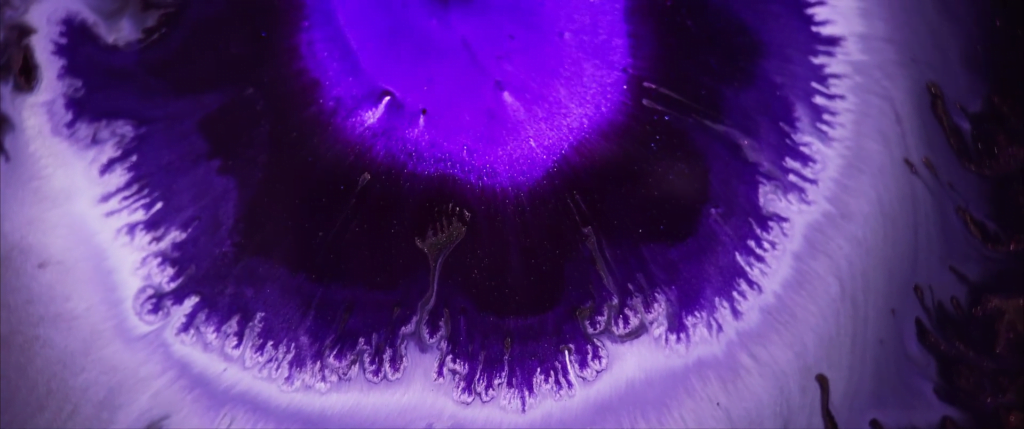
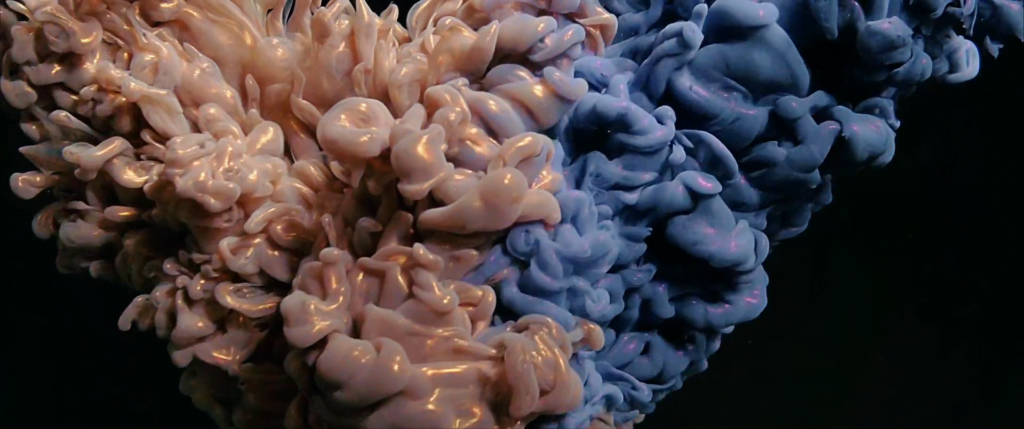
Billowing turbulence, mushroom-like Rayleigh-Taylor instabilities, and spreading flows abound in Vadim Sherbakov’s “Origin.” The short film takes a macro looks at fluids — inks, alcohols, soaps, and other household liquids. It was filmed entirely on a DJI Pocket 2, a rather small, stabilized pocket camera. It’s a testament to what you can achieve with some experimentation and relatively inexpensive equipment. (Video and image credit: V. Sherbakov)

Convection in Action
We’re surrounded daily by convection — a buoyancy-driven flow — but most of the time it’s invisible to us. In this video, Steve Mould shows off what convection really looks like with some of his excellent tabletop demos. The first half of the video gives profile views of turbulent convection, with chaotic and unsteady patterns. When he switches to oil instead of water, the higher viscosity (and lower Reynolds number) offer a more structured, laminar look. And finally, he shows a little non-temperature-dependent convection with a mixture of Tia Maria and cream, which convects due to evaporation changing the density. (Image and video credit: S. Mould; submitted by Eric W.)

Serpents and Ouroboros
Beads of condensation on a cooling, oil-slicked surface have a dance all their own in this video. Large droplets gobble up their fellows as they follow serpentine paths; each new droplet donates its interfacial energy to feed the larger drop’s kinetic energy. Eventually, the big drops switch to a circular path, like an ouroboros, the tail-eating serpent of mythology. This transition happens due to the oil shifted by the dancing droplets. You can recreate the effect at home by rubbing a thin layer of oil over glass and setting it atop a hot mug of your favorite beverage. (Video and image credit: M. Lin et al.; research credit: M. Lin et al.)

Food-Based Fluid Dynamics
The kitchen is a rich source of fluid physics. From cocktails to coffee, from crepes to tempura, food is full of physics. In fact, it’s not hard to relate almost any fluid phenomenon you can imagine to something that goes on in the kitchen. That’s why scientists managed to write a 77-page review article of culinary fluid dynamics. It’s even structured after a menu, carrying readers from the kitchen sink and cocktails all the way through a meal and the process of washing up afterward! (Image credit: top – S. Hsu, others – A. Mathijssen et al.; research credit: A. Mathijssen et al.; via APS Physics)