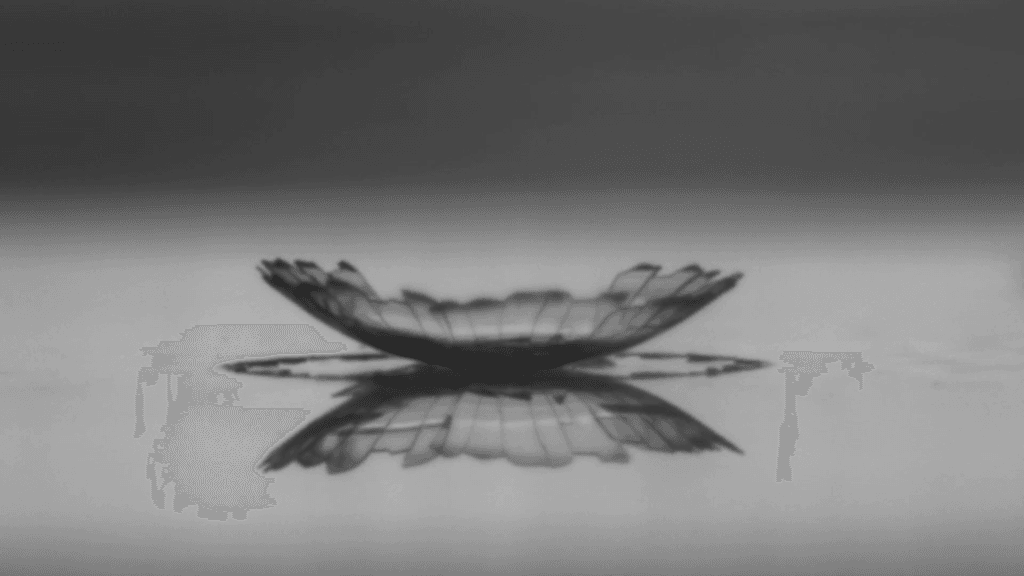
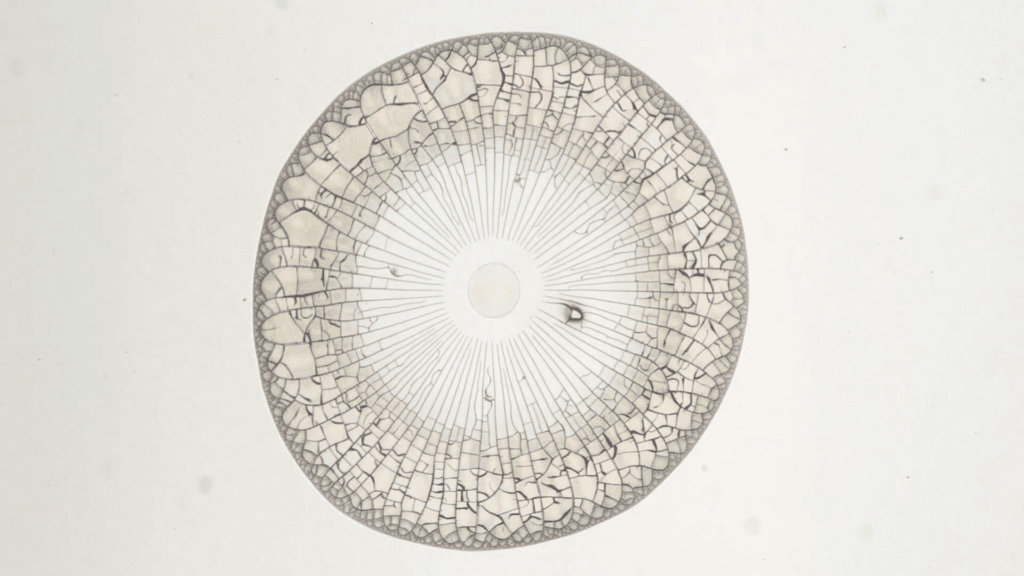
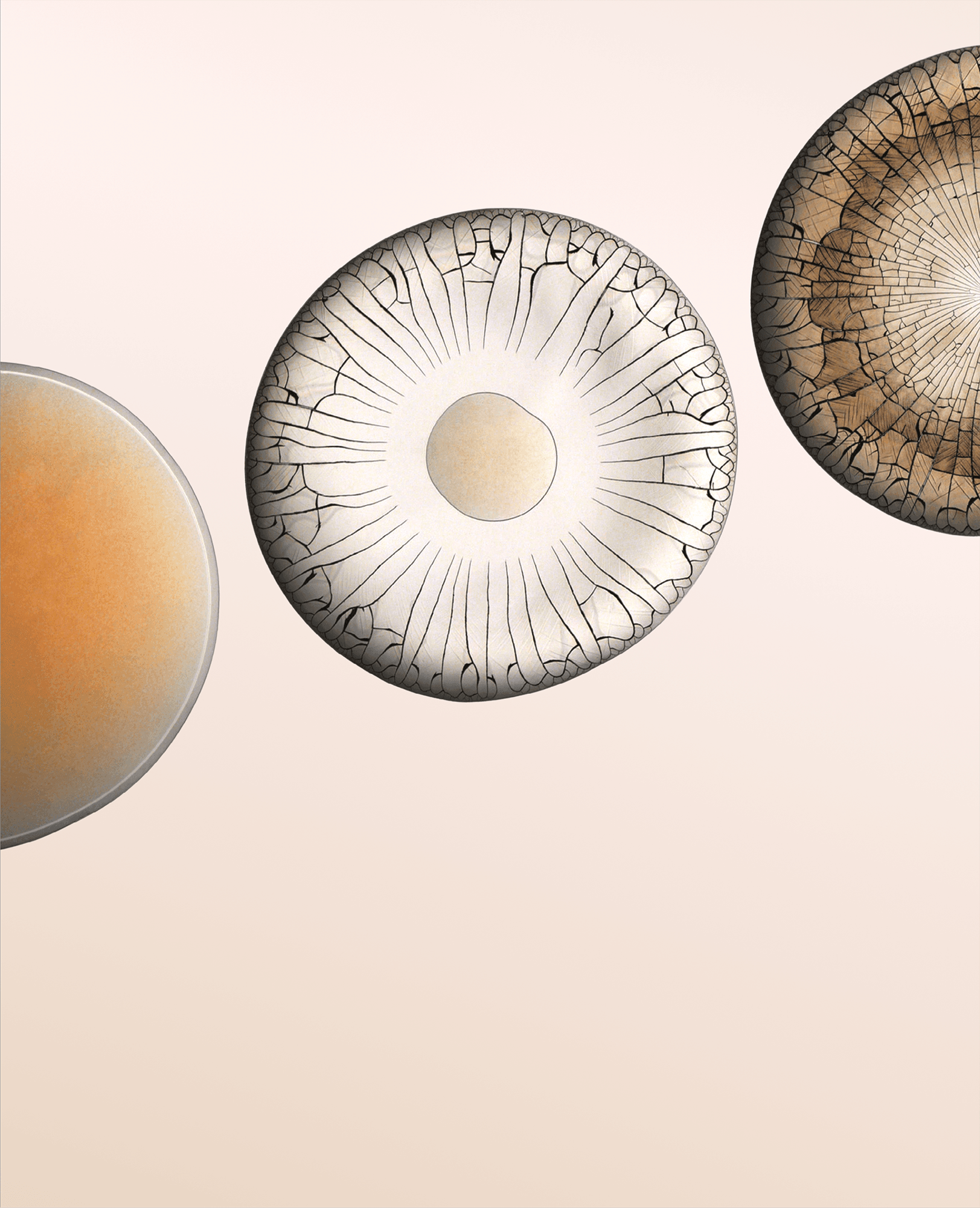Water droplets filled with nanoparticles leave behind deposits as they evaporate. Like a coffee ring, particles in the evaporating droplet tend to gather at the drop’s edge (left). As the water evaporates, the deposit grows inward (center) and cracks start to form radially. After just a couple minutes, the solid deposit covers the entire area of the original droplet and is shot through with cracks (right).
Researchers found that the cracks’ patterns and propagation are predictable through a model that balances the local elastic energy and and the energy cost of fracture. They also found that the spacing between radial cracks depends on the deposit’s local thickness. Besides explaining the patterns seen here, these cracking models could help analyze old paintings, where cracks could hide information about the artist’s methods and the artwork’s condition. (Image and research credit: P. Lilit et al.; via Physics Today)