There are many ways to make a droplet oscillate in a star-shape – like vibrating its surface or using acoustic waves to excite it – but these methods involve externally forcing the droplet’s oscillation. Leidenfrost drops – liquids levitating on a film of their own vapor caused by the extremely hot surface below – turn themselves into stars. It all starts with the constant evaporation driven by the heat below. This creates a thin, fast-moving layer of vapor flowing beneath the drop. That vapor shears the drop, causing capillary waves – essentially ripples – that travel through the drop in a characteristic way. Those ripples in turn cause pressure oscillations in the vapor layer, alternately squeezing and releasing it. Feedback from the vapor layer then drives the droplet into star-shaped oscillations. Under the right conditions, water drops can form stars with as many as 13 points! (Image and research credit: X. Ma and J. Burton, source)
Tag: Leidenfrost effect

Rolling Along
Leidenfrost drops – droplets deposited onto a surface much hotter than their boiling point – are known for their mobility. With the right surface, they can be propelled, trapped, and even guided through a maze, typically by directing the vapor layer that cushions them. But new work shows that these drops have internal dynamics that also contribute to their propulsion.
By adding tracer particles to each droplet, researchers can visualize flows inside the droplet. Large drops tend to have a flatter shape and contain two or more rotating vortices. Such drops won’t propel themselves without another force in play. But smaller droplets are more spherical and contain only a single rotating flow. Once these drops detach, they roll away! Despite the similarity to wheels, these liquid drops aren’t moving the same way. Remember that the drop is not actually in contact with the surface. To see what sets the drop’s direction, researchers examined the shape of the bottom of the drop. They found that it sits at a slant on its vapor cushion. That pushes evaporating gases out one side, propelling the drop the other way. (Image and video credit: A. Bouillant et al., source)

Controlling Leidenfrost Drops
On a surface much hotter than their boiling point, droplets can surf on a layer of their own vapor due to the Leidenfrost effect. Recent research has shown that textured surfaces like ratchets can create corrals, traps, and mazes for such droplets. Here, researchers manipulate the propulsion of Leidenfrost drops using non-parallel grooves instead. When placed between two non-parallel plates, the droplet is squeezed by side forces perpendicular to the walls, with the resultant force in the direction where the gap widens. In most states, friction forms an opposition to this squeeze, but for Leidenfrost droplets that frictional force is negligible. Instead, the squeezing from the plates launches droplets toward the wider end of the groove, allowing researchers to design repellers (top) and traps (bottom) for the fast-moving drops. (Image credits: C. Luo et al., source)

Self-Propelled Hovercraft

When placed on an extremely hot substrate, some drops levitate and can be propelled over specially textured surfaces. Inspired by this work, researchers are using similar principles to explore manipulation of levitating plates using surface texture. Their apparatus consists of a semi-porous, grooved surface that ejects air upward to levitate Plexiglas objects – think air hockey table with grooves. With enough airflow, the Plexiglas levitates. The grooves force air in a particular direction – in the case of the herringbone pattern, this is in the direction of opening – and, as the air moves, it drags its Plexiglas hovercraft along. As shown in the second animation, grooves can do more than move the glass linearly; with patterns offset by 90-degrees, they can make the hovercraft rotate.
Here’s an interesting next step for anyone out there with an air hockey table and a 3D printer: does the directional manipulation work if the grooves are on the object and not the table? In other words, can you create an air hockey puck that preferentially goes to your opponent’s goal? (Image and resource credit: D. Soto et al., source)

Self-Propelling Drops
Droplets of acetone deposited on a bath of warm water can float along on a Leidenfrost-like vapor layer. The droplets are self-propelling, too, thanks to interactions between the acetone and water. Acetone can dissolve in water, and when acetone vapor beneath the drop gets absorbed into the water bath, it lowers the local surface tension. That drop in surface tension creates a pull in the direction of a higher surface tension; this is what is known as the Marangoni effect. Because of that flow in the direction of higher surface tension, the acetone drop accelerates away. (Image credit: S. Janssens et al., source)

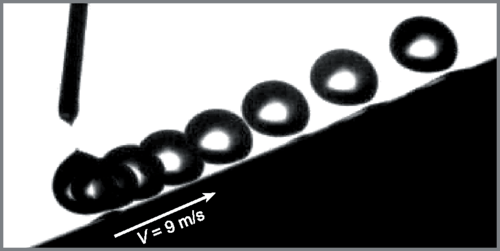
Aerodynamic Leidenfrost Effect

If you place a droplet on a surface much hotter than its boiling point, that droplet will skitter and float almost frictionlessly across the surface on a thin layer of its own vapor. This is what is known as the Leidenfrost effect. But you don’t have to heat a surface to get this behavior. There’s also an aerodynamic Leidenfrost effect, shown above, when the surface is moving. As the surface moves, it drags a layer of air along with it, and that layer of air is capable of keeping droplets aloft indefinitely. The thickness of the air layer depends on speed; the faster the plate moves, the thicker the air layer underneath droplets. The aerodynamic forces generated are large enough to drive a droplet up an incline against the force of gravity (bottom image). (Image credit: animation – M. Saito et al., source; chronophotograph – A. Gautheir et al., pdf)

Leidenfrost Atop a Fluid
Leidenfrost droplets typically hover on a thin layer of vapor above a surface that is much hotter than the boiling point of the liquid. Such drops move almost frictionlessly across these surfaces and can even propel themselves. The question of how hot is hot enough to produce the Leidenfrost effect is still being debated, but recent research suggests that the answer may depend strongly on surface roughness.
To test the role of surface roughness, one group tested drops of ethanol atop a heated pool of silicone oil, as pictured above. Ethanol’s boiling point is 78 degrees Celsius, and the researchers found they could hold the ethanol drop in a Leidenfrost state by heating the pool to 79 degrees Celsius – only 1 degree above ethanol’s boiling point! Thanks to surface tension, a liquid surface is essentially molecularly smooth. The fact that solid surfaces require much higher temperatures before the Leidenfrost effect is observed indicates that even the slightest roughness can have a large impact on the Leidenfrost temperature. (Image credit: F. Cavagnon; research credit: L. Maquet et al., pdf)
Heads-up for Boston-area folks! I’ll be taking part this Saturday evening in the Improbable Research show at the AAAS conference. The show is free and open to the public but fills up quickly, so be sure to come early for a seat.

Surfing on Vapor
Place a drop of liquid on a surface much, much hotter than the liquid’s boiling point, and the portion of the drop that impacts will vaporize immediately. This leaves the droplet hovering on a thin layer of vapor. With a fluid like water, the vapor state is a much more efficient insulator than the liquid state. Thus, the vapor layer actually protects the liquid droplet, enabling it to boil off at a much slower rate than if the drop were touching the heated surface. This is known as the Leidenfrost effect, and it can be used to create self-propelled droplets. (Image credit: R. Thévenin and D. Soto)

Leidenfrost Atop Gasoline

The animations above show a little of what happens when you pour a spoonful of liquid nitrogen onto a container of gasoline. A couple of things are happening simultaneously here. First of all, the liquid nitrogen is experiencing the Leidenfrost effect. Because of the extreme difference in temperature between the gasoline (~20 degrees C) and the liquid nitrogen (-196 degrees C), part of the nitrogen is evaporating immediately, creating a vapor layer that insulates the remainder of the liquid nitrogen and allows it to float above the gasoline surface. The same thing happens to water drops on a very hot skillet.
The extreme cold of the nitrogen also seems to have formed some ice that’s further protecting the nitrogen drop. I’m not 100% sure what that would be made of, though – a mixture of water and gasoline?
Finally, there’s the simultaneous evaporation of the liquid nitrogen and the sublimation of the ice. This is the white vapor we see propelling and spinning the ice/drop. Note the “bounce” that happens in the top animation. The drop never actually impacts the wall. When it gets close, the escaping vapors are affected by the wall and start pushing the drop in a new direction! Check out the whole video below. (Image credit: carsandwater; via Gizmodo)

Daily Fluids, Part 3
A lot of the fluid dynamics in our daily lives centers around the preparation and consumption of food. (And in its digestion afterward, but that’s another story!) Here are a few examples of fluid dynamics you might not have realized you’re an expert on:

Low Reynolds Number Flows
This is a fancy way of discussing the motion of syrup, honey, and other thick and viscous fluids we interact with in our lives. These flows are typically slow moving and exhibit some neat properties like coiling or being possible to unstir.
Immiscible Fluids
Oil and water don’t mix, a fact anyone familiar with salad dressings or marinades is well aware of. The way around this is to shake them up! This disperses droplets of the oil within the water (or vinegar or whatever) to create an emulsion. While not truly mixed, it does make for more pleasant eating.
Multiphase Flows
Multiphase flows are ones containing both liquid and gaseous states. Boiling is an example we often see in our daily lives, though carbonated beverages, water sprayers, and sneezes are other common ones.
Leidenfrost Effect
The Leidenfrost effect occurs when liquid is introduced to a surface that is much, much hotter than its boiling point. Part of the liquid instantly vaporizes, leaving droplets to skitter around on a thin vapor layer. This is most often seen around the stove and in skillets. (And, yes, it does qualify as a multiphase flow!)Tune in all week for more examples of fluid dynamics in daily life. (Image credit: S. Reckinger et al., source)
P.S. – I’m at VidCon (@vidconblr) this year! If you are, too, come say hi and get an FYFD sticker 😀