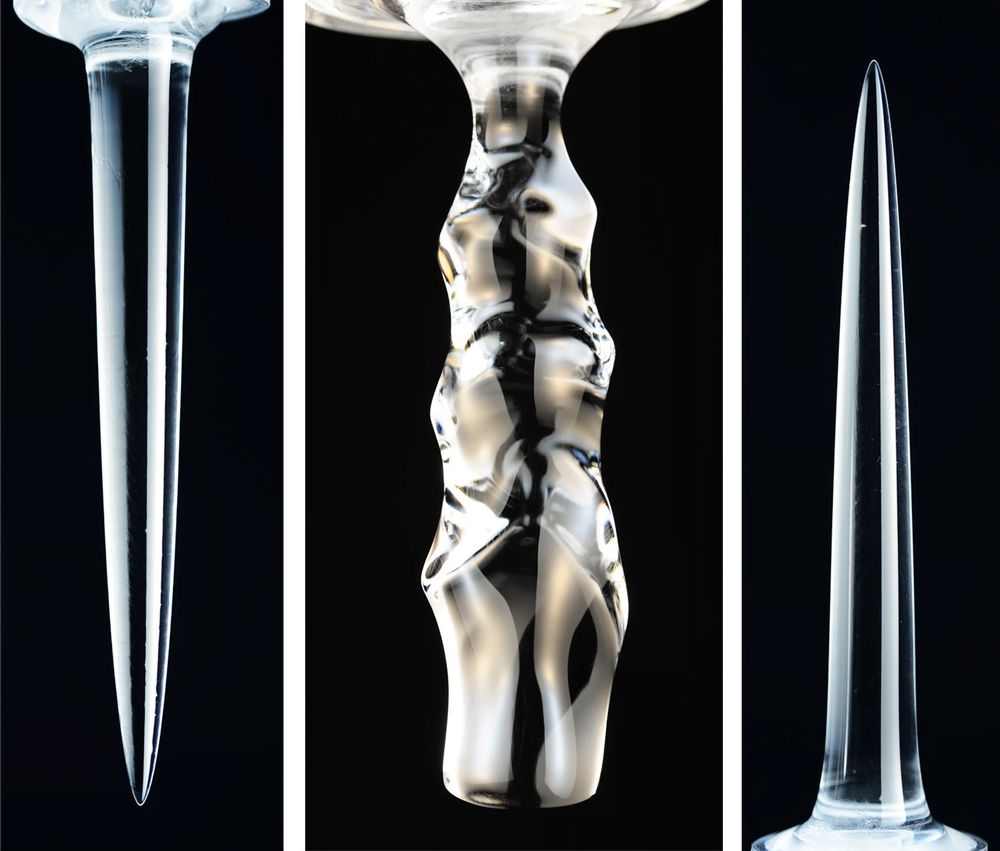
Water is an odd substance because it is densest at 4 degrees Celsius, well above its melting point at 0 degrees Celsius. This density anomaly means that melting ice takes on very different shapes, depending on the temperature of the water surrounding it. At low temperatures (under 4 degrees Celsius), the cold water melting off the ice is denser than the surroundings, so it sinks. The sinking fluid melts lower portions of the ice faster, leading to an inverted pinnacle (Image 1).
In contrast, at higher temperatures (above 7 degrees Celsius), the meltwater is lighter than the surroundings and therefore rises, creating an upward-pointing pinnacle (Image 3). At intermediate temperatures, some areas of the ice see rising meltwater and some see sinking. This complicated flow pattern sets up vortices that result in a scalloped edge along the ice (Image 2). (Image and research credit: S. Weady et al.; via APS Physics)



Leave a Reply