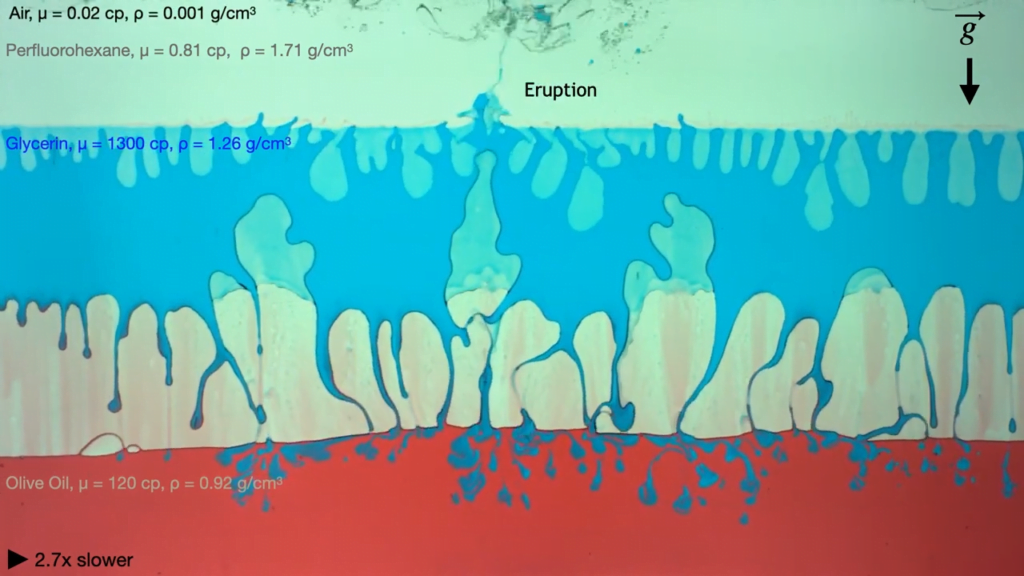
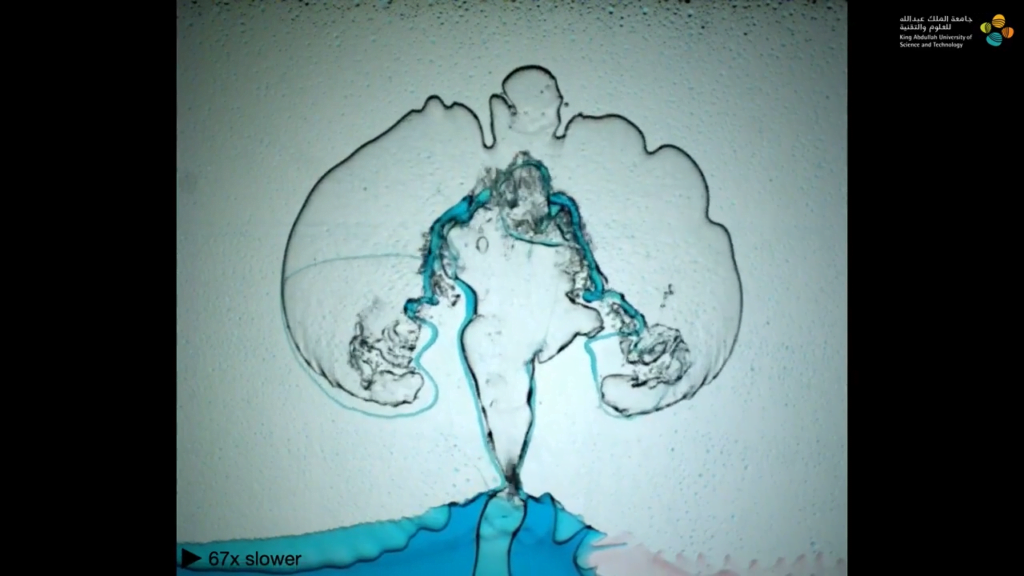
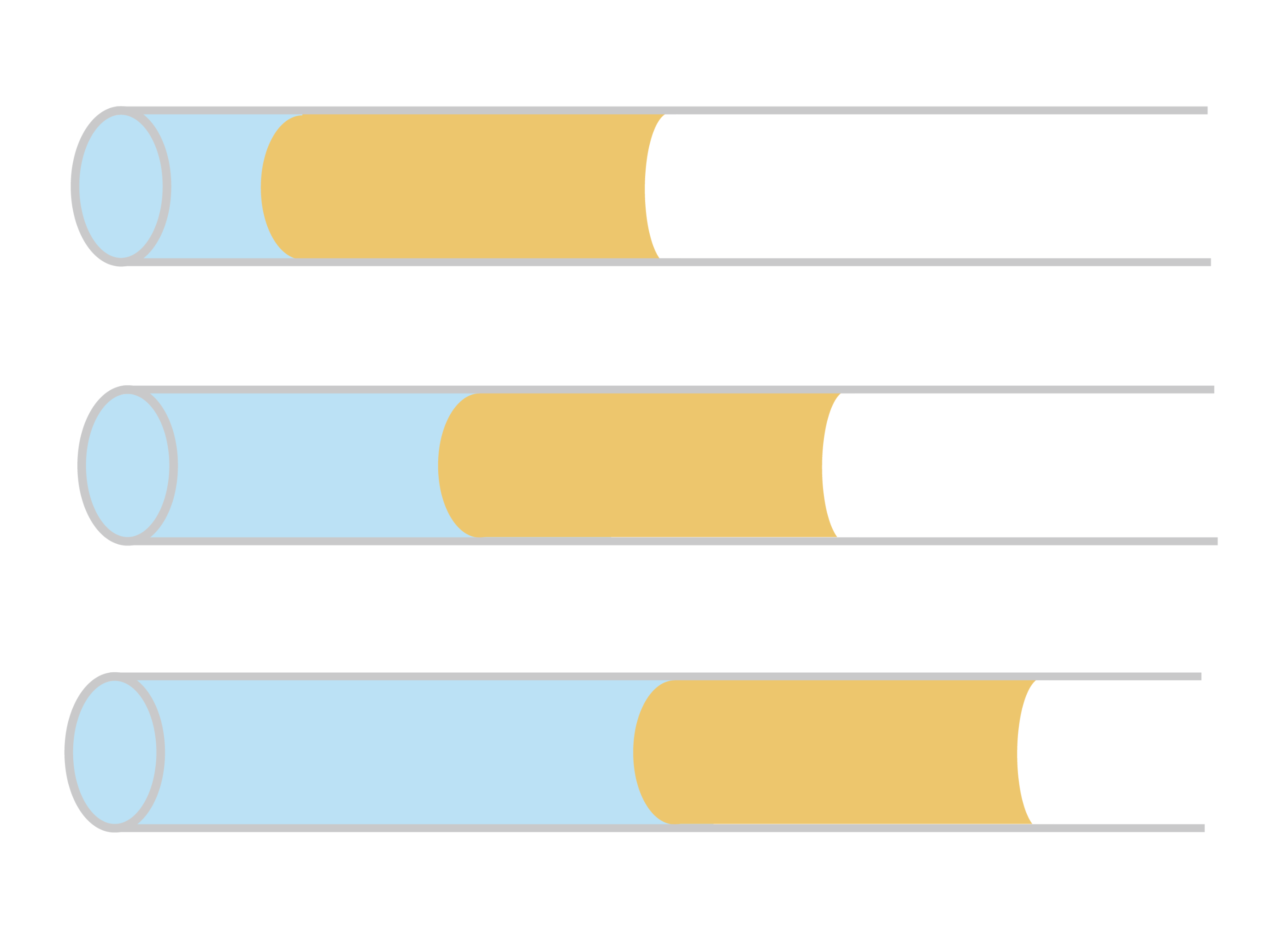
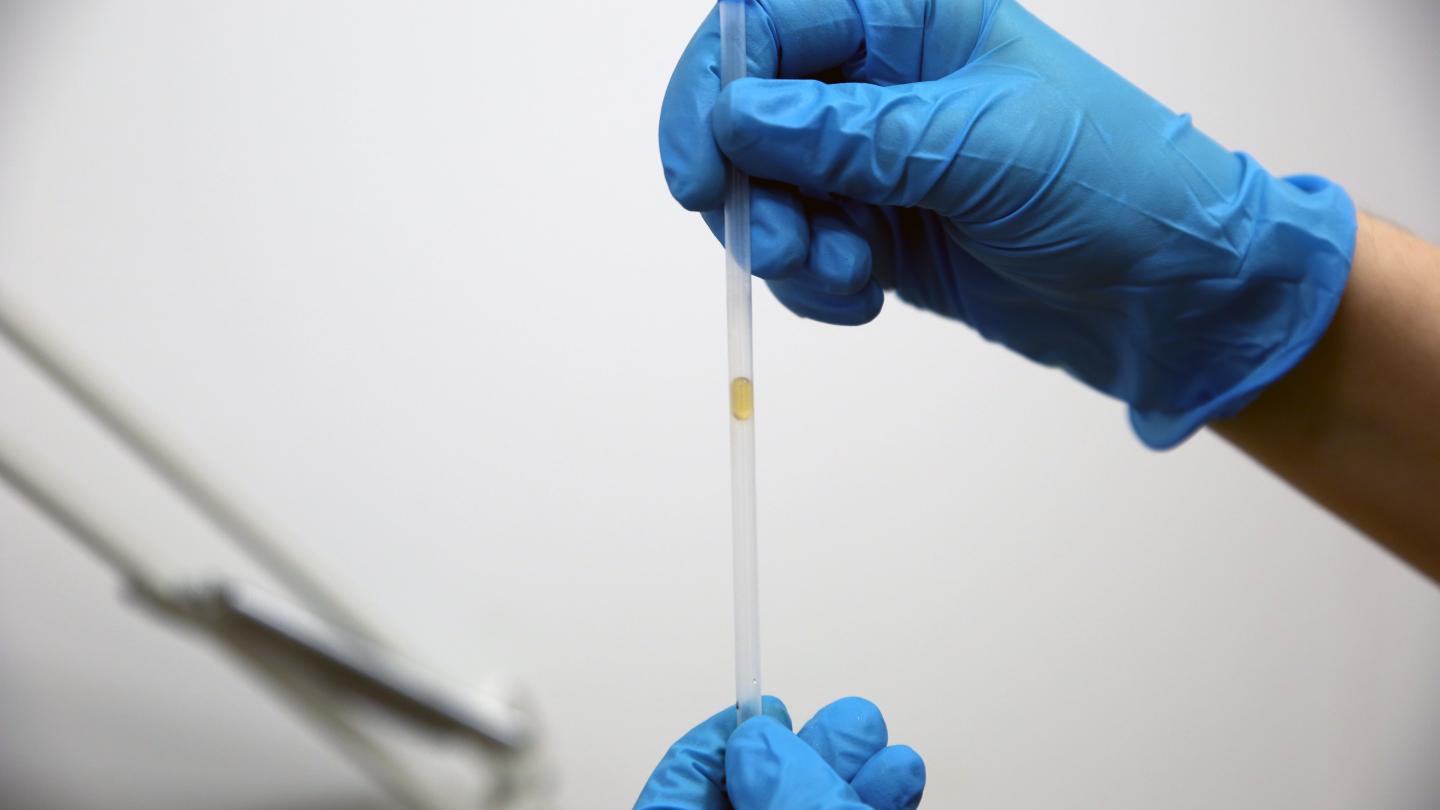
Busy bees feed on millions of flowers for each kilogram of honey they produce. To gather nectar, bees use their hairy tongues, which project out of a sheath-like cover. Protraction (i.e., sticking their tongue out) is relatively fast because all the hairs on the tongue initially lie flat. In the nectar, those hairs flare out, creating a miniature forest that traps viscous nectar and drags it back into the bee during retraction.

Through modeling and experiments, researchers found that the time it takes a bee to retract its tongue depends on the bee’s overall mass. Smaller bees are slower to the retract their tongues, likely to allow enough time for their shorter tongues to capture enough nectar. With bee populations on the decline, the team’s predictions may help communities select flowers with nectar concentrations that best fit their local bees’ needs. (Image credits: top – J. Szabó, bee eating – B. Wang et al.; research credit: B. Wang et al.; via APS Physics)