Many insects and arachnids can walk on water by virtue of their hydrophobicity and small size. With their light weight and skinny legs, these invertebrates curve the air-water interface like a trampoline, with surface tension providing the elasticity that keeps them afloat. What’s truly incredible, though, is that many of these creatures, like water striders, can actually jump off the water surface.
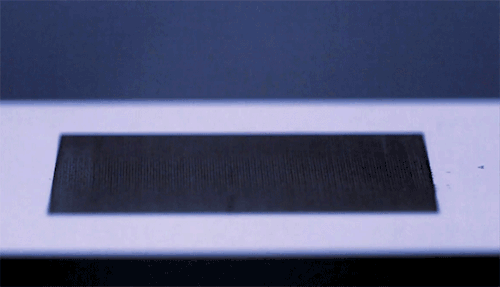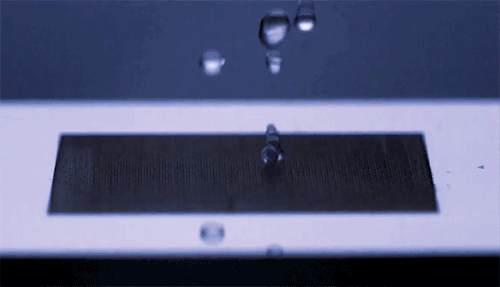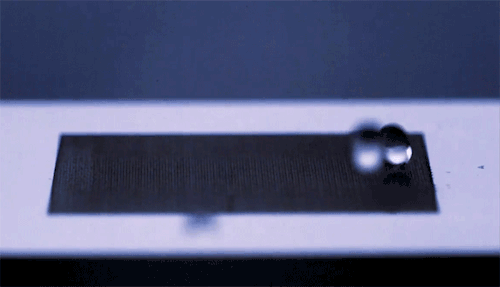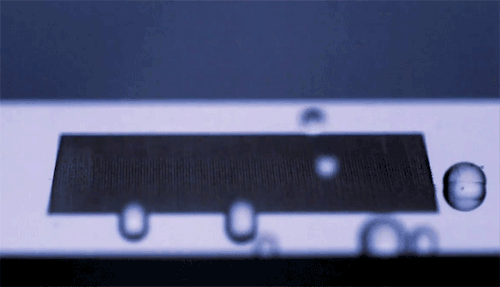
The top animation shows high-speed video footage of a water strider leaping off the water. Notice how it distorts the air-water interface but doesn’t break the surface – it makes no splash.
The key is not to push too hard. If the insect exerts a force exceeding the limits of what surface tension can withstand, then its legs will break the water surface and it will lose energy to drag and viscous forces. The insect must generate its jumping force without exceeding a hard limit.
The water strider achieves this feat not by pushing downward but by rotating its middle and hind legs. Rotating its legs allows the insect to maintain contact with the water surface longer and continue deforming the interface as it jumps. This maximizes the momentum it transfers to the water, which, in turn, increases the insect’s take-off velocity. By studying and then emulating this mechanism, scientists were able to successfully create a tiny 68-mg water-jumping robot. (Image credits: J. Koh et al., sources, PDF)
This week FYFD is exploring the physics of walking on water, all leading up to a special webcast March 5th with guests from The Splash Lab.