Leidenfrost drops surf on a layer of their own vapor, created by the high temperature of a nearby surface relative to their boiling point. These Leidenfrost drops can self-propel and skitter and skate across a surface, but they’re not the only droplets that do this. In this video, researchers show how a drop of carbonated water on a superhydrophobic (water-repelling) surface also avoids contact. As long as the drop has carbon dioxide to expel, it will maintain a gap relative to the surface and can even surf over a ratcheted surface the way that their Leidenfrost cousins do. (Image and video credit: D. Panchanathan et al., source)
Tag: superhydrophobic

Jumping Droplets
From butterfly wings to lotus leaves, many surfaces in nature are shaped to repel water. This typically means roughness on the scale of tens of nanometers, which helps trap air between water and the surface. Droplets can still form on these surfaces, but when they merge, the sudden excess of surface energy sends the coalesced droplet flying. With enough height, the tiny droplet can catch the wind and get carried away. It’s like a natural anti-fogging mechanism, and it’s one that engineers are keen to understand and replicate. (Image and research credit: P. Lecointre et al.)

Moving Droplets
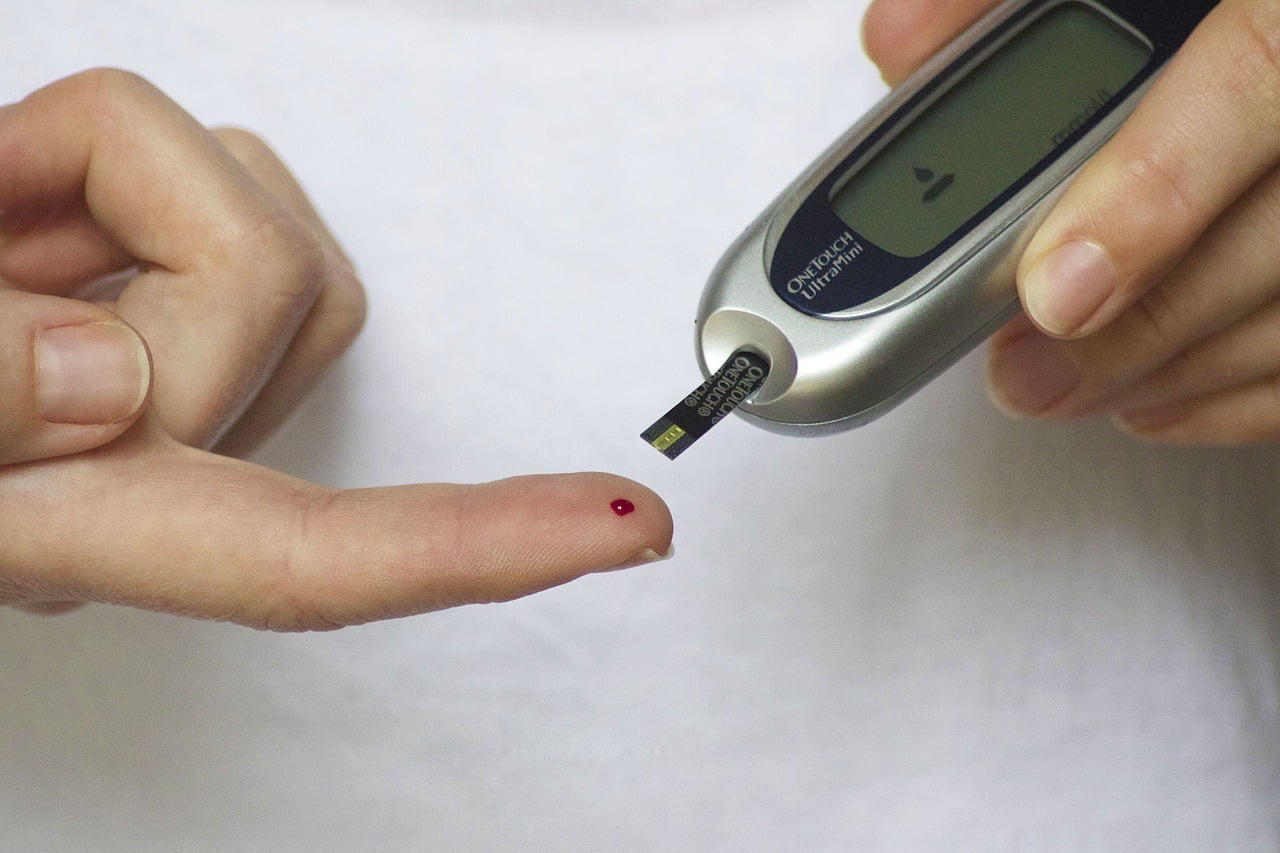
Microfluidic devices – such as those used by individuals with diabetes to monitor their blood glucose levels – are all about transport. Typically, these devices use some kind of externally applied force, like a temperature gradient or electrical field, to force liquids through the device’s narrow channels. But a new study describes a way to move droplets without an external force.
The researchers built their devices using two slips of glass, coated with an oil-attracting, water-repellent mixture. They attached the glass slips with a narrow spacer at one end, leaving the other end free. This made a narrow, but slightly flexible gap. When the scientists placed an oil drop inside the closed end, it spread on the glass, pulling the two sides closer to one another. Water drops, on the other hand, tried to force the walls apart, in an effort to minimize contact. Both sets of drops, interestingly, moved toward the open end of the device.
The researchers found that the shapes assumed by the droplets create an internal pressure gradient, which, in both cases, slowly moves the drops. They call this method bendotaxis, a type of self-propulsion driven by the drops’ ability to bend the material they’re touching. It’s not a fast way to transport fluids – the drops moved only a few micrometers per second – but it may be useful for applications like drug deliveries where the liquid needs to be administered slowly over a longer period. (Image credit: TesaPhotography; research credit: A. Bradley et al.; via APS Physics; submitted by Kam-Yung Soh)

Putting a Spin on Splashes
Researchers put a spin on splashing droplets with selective wetting. When a drop impacts on a water-repellent, superhydrophobic surface, it will spread circularly, then pull back together and rebound off the surface. That’s because the surface coating resists actually touching – or being wetted by – the water. But just as there are surface coatings that resist water, there are those that attract it.
Above, researchers have coated a surface so that it’s mostly superhydrophobic, but it also has narrow pinwheel-like arms that are hydrophilic. As the drop impacts, it spreads across the surface and then retracts. But where the hydrophilic arms are, the drop lingers. This creates the four lobes we see on the droplet, and the asymmetric retraction gives the drop angular momentum. As it leaves the surface, the spin continues. In some configurations, the researchers could make the drop spin at more than 7300 rpm. (Image and research credit: H. Li et al; via Science; submitted by Kam-Yung Soh)

Avoiding Ice
Keeping ice from forming on a surface is a major engineering challenge. Typically, there’s no controlling certain factors – like the size and impact speed of droplets – so engineers try to tame ice by changing the surface. This can be through chemicals – as with deicing fluids used on aircraft – or by tuning the surface itself.
One way to do this is by making the surface superhydrophobic – or extremely water repellent. These surfaces are rough on a nanoscale level, but they’re delicate, and once ice gets a grip on them, it’s even harder to remove. In a recent study, however, researchers used particles with both hydrophobic and hydrophilic – water-attracting – properties to create a superior ice-resistant surface. The combination of hydrophobic and hydrophilic aspects to the particles made supercooled droplets break up on contact with the surface. This made the drops smaller and decreased their contact time, making it harder for them to stick and freeze. (Image credit: Pixabay; research credit: M. Schwarzer et al.; via Chembites; submitted by Kam-Yung Soh)

Water Anoles Breathe Underwater
Meet the water anole, a small lizard native to the tropics of Central America. While studying these anoles, researchers discovered that they could flee underwater and remain submerged for 16 minutes or more at a time. Curious to see how the lizard manages this feat, they filmed them underwater, discovering that the anole seems to exhale a small bubble that sticks on its face and then re-inhale it.
How exactly this built-in “scuba gear” works is still under investigation, but here’s my guess. Fresh oxygen can diffuse from water into a bubble; some insects use this to breathe underwater. The natural, random motion of molecules tends to cause chemicals to move from areas of high concentration to those of low concentration. But this molecular diffusion is extremely slow. That tiny bubble you see isn’t around long enough for any significant molecular diffusion of fresh oxygen. But what if the surface of the bubble is actually much larger?
Notice the silvery shininess we see on the anole. That’s because most of the lizard isn’t actually wet. The anole is superhydrophobic, so its skin has trapped a thin layer of air that appears to extend over a large part of its body. I think perhaps the anole has fresh oxygen diffusing into the air layer across most of its skin, and the large bubble it inhales and exhales serves as a sort of pump to help draw that fresh oxygen through the air layer and into its body. That could help explain how the anole can stay submerged for so long.
As researchers continue to investigate this little aquanaut, it will be interesting to discover just what its secrets are! (Image and video credit: L. Swierk; via Gizmodo)

Water-Walking Geckos
Many animals can run on water. The tiniest creatures, like water striders, use surface tension to keep themselves atop the water. Larger creatures like the basilisk lizard or the grebe slap the water’s surface to generate a vertical impulse that keeps them aloft. Geckos, it turns out, can run on water, too, but they’re too big to stay up with surface tension and too small to support their weight by slapping. So they’ve developed their own method.
As you see in the top image, geckos use the slapping method for part of their support. Their slaps generate a little less than half of the force needed to keep them out of the water.
Surface tension is an important component, too. Geckos are extremely water repellent, which helps boost the lift they get from surface tension. In the bottom image, you see a gecko attempting to run on soapy water, which has a lower surface tension. The gecko is mostly submerged and more swimming than running – a clear demonstration that surface tension is important to its water-walking.
Finally, the gecko undulates its body as it runs, much the way an alligator swims. The researchers suspect this helps the gecko generate forward thrust. Altogether, it creates a water-walking gait that, for now, is unique among observed mechanisms. (Image and research credit: J. Nirody et al.; via Ars Technica; submitted by Kam-Yung Soh)

Surfaces That Scrape Off Ice
Ice can be a terrible pest, freezing to surfaces like roads and airplane wings and causing all sorts of havoc. Some surfaces, though, can actually prompt a freezing drop to scrape itself off. There are a couple key effects in play here. The first is that the surface is nanotextured – in other words, it has extremely small structures on its surface. This makes it hydrophobic, or water-repellent. The second key ingredient is that the drop is cooling evaporatively; that means heat is escaping along the air-water interface instead of conducting through the solid surface. As a result, the freezing front forms at the interface and pushes inward. Water expands as it freezes, which tries to force the interior liquid out, toward the bottom of the drop. On a normal surface, this would force the contact line – where air, water, and surface meet – to push outward. But the nanotexture of the hydrophobic surface pins that line in place. So the expanding ice pushes the frozen drop upward, scraping it off the surface! (Video and image credit: G. Graeber et al., source)

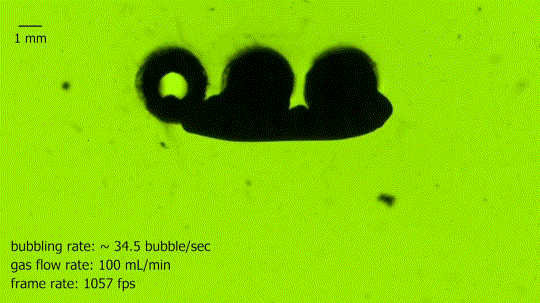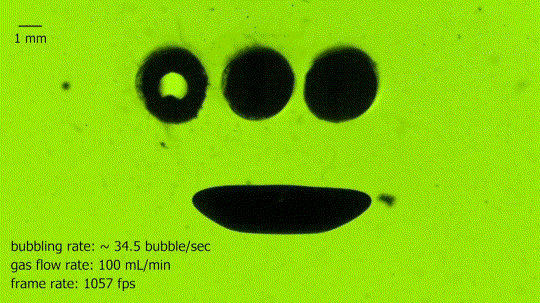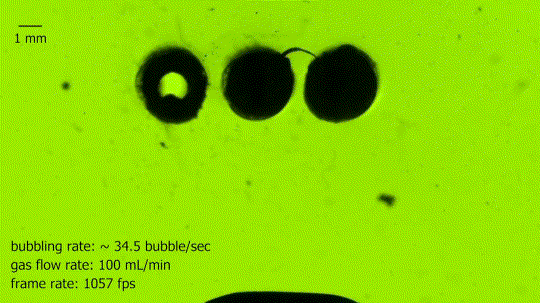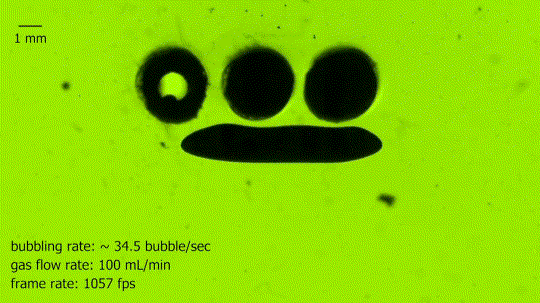
Absorbing Bubbles
This is a bubble absorber. It’s formed from an array of three springs, seen end-on in the upper center, each of which is coated to make it superhydrophobic. The hollow interior of the springs is filled with air and ventilated to the atmosphere. As bubbles rise through the water, they contact the springs and readily coalesce with the interior gas. In the blink of an eye, the large bubble is almost completely absorbed into the thin air film that clings to the springs. Superhydrophobic arrays like these may be useful in power and life support systems that need to separate liquid and gas phases under low-gravity conditions. (Image credit: N. Pour and D. Thiessen, source)

Jumping Droplets
Condensation, which removes heat by changing a vapor into a liquid, is a common feature in industrial heat transfer. When droplets form on surfaces, they typically have to grow to millimeter size before gravity causes them to slide off and open up the surface to new droplet formation. Hydrophobic surfaces can shed droplets a little sooner. Droplets only 100 micrometers in size will spontaneously jump off hydrophobic surfaces due to the release of excess surface energy during droplet coalescence, but this only happens when those droplets have a small contact area with the surface. Defects in the nanoscale structure of the surface can allow water to squeeze in between posts and hold on.
To counter this, new experiments packed copper nanowires into a dense 3D array. This permits fewer defects and helps condensing droplets leap from the surface sooner. Each droplet carries away a bit of the surface’s heat. The new method is impressively efficient at it. Researchers found the new heat exchanger could remove 100% more heat than previous hydrophobic designs. (Video credit: Science; research credit: R. Wen et al.)