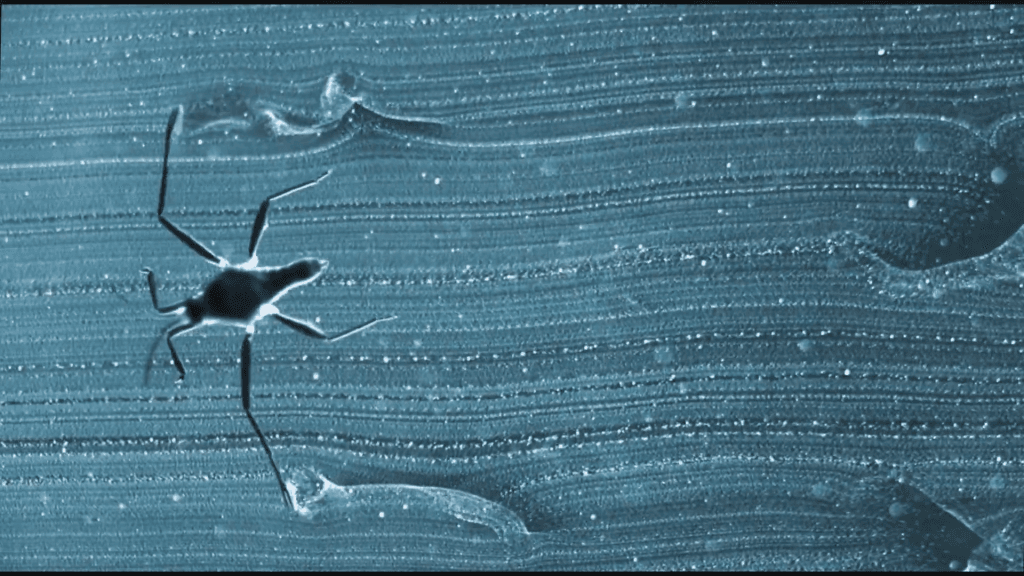
Ripple bugs are a type of water strider capable of moving at a blazing fast 120 body lengths per second across the water surface. In addition to their speed, ripple bugs are incredibly agile and are active almost constantly. Researchers believe they’ve found the insect’s secret: feather-like hydrophilic fans that spread on contact with the water. These fans help the insects push off the water and steer, but they require no effort to open and close. They’ve even adapted the technique to bio-inspired robots and seen improvements in speed, agility, and efficiency. (Video credit: Science; research credit: V. Ortega-Jimenez et al.)
Tag: hydrophilic

Drops of Fiber Suspensions
To 3D print with fiber-infused liquids, we need to understand how these drops form, break-up, and splash. That’s the subject of this research poster, which shows drops of a fiber suspension forming and pinching off along the top of the image. In the lower half of the image, drops of the suspension hit a hydrophilic surface and spread. How the drop and its fibers spread will affect the final properties of the printed material. (Image credit: S. Rajesh and A. Sauret; via GoSM)

Drag Is Greatest Before Submersion
A new study shows that partially submerged objects can experience more drag than fully submerged ones. This unexpected result comes from the excess fluid that piles up ahead of the object, as seen in the image above, where flow is moving from left to right. The experiments used centimeter-sized spheres and showed that the maximum drag on a nearly-submerged sphere could be 300-400% greater than the drag on a fully submerged sphere.
Even more surprisingly, they found that water-repellent hydrophobic coatings — which are often suggested for drag reduction — actually increased the drag even further on partially submerged spheres. That’s because the water-repelling coating caused an even larger build-up of fluid ahead of the sphere, increasing the pressure on the front side of the sphere and creating even more drag. Spheres with a hydrophilic coating had less water build-up and thus lower drag.
The study suggests that — at the centimeter-scale — drag physics at the air-water interface may be more complicated than we assume. (Image and research credit: R. Hunt et al.; via Physics World; submitted by Kam-Yung Soh)

Aquatic Escape Artists
Springtails are tiny hexapods found living on the air-water interface. Like other creatures living at the interface, they sometimes need to make a quick escape. For the springtail, that means a high-flying leap, driven by their fork-shaped furcula. The springtail soars into the air, where it contorts its body and uses aerodynamic forces — along with a droplet it carries on its belly — to orient itself. For landing, it uses that droplet as a sticky anchor that helps it adhere to water (or ground) instead of bouncing. Nailing that landing sets it up to make another daring escape as quickly as needed. (Video and image credit: Deep Look; research credit: V. Ortega-Jimenez et al.)

The Two-Faced Splash
The way a sphere enters water depends on its size, speed, and surface properties. A hydrophilic (water-attracting) sphere behaves differently than a hydrophobic (water-repelling) one. But what happens when the object’s surface properties aren’t uniform?
That’s the situation we see above. The dark line marks the two hemispheres of the sphere and their differing surface properties. To the left, the sphere is hydrophilic; to the right, it is hydrophobic. When the sphere hits the water, both the splash and underwater cavity quickly become asymmetric. On the hydrophobic side, the cavity wall is smooth, but the cavity is rough on the hydrophilic side. In the end, the asymmetries create a horizontal force that pushes the sphere sideways. (Image and research credit: D. Watson et al.)

Bouncing Off Hydrophilic Surfaces
Droplets typically bounce off hydrophobic surfaces due to air trapped beneath the liquid that prevents contact between the drop and surface. But even extremely smooth, hydrophilic surfaces can elicit a bounce under the right circumstances, as shown in a new study.
The key is that the droplet must bounce at exactly the right speed. If the bounce has too much momentum, it will squeeze the nanometer-sized air cushion too thin, allowing contact. Too slow and the Van der Waals attraction between the droplet molecules and wall molecules will have time to act. But between those lies a sweet spot where the dimple and cushion of air beneath the drop keep it from impacting. (Image credit: droplets – klickblick, drop bounce – J. Kolinski, bounce sim – J. Sprittles et al.; research credit: M. Chubynsky et al.; submitted by James S.)

Putting a Spin on Splashes
Researchers put a spin on splashing droplets with selective wetting. When a drop impacts on a water-repellent, superhydrophobic surface, it will spread circularly, then pull back together and rebound off the surface. That’s because the surface coating resists actually touching – or being wetted by – the water. But just as there are surface coatings that resist water, there are those that attract it.
Above, researchers have coated a surface so that it’s mostly superhydrophobic, but it also has narrow pinwheel-like arms that are hydrophilic. As the drop impacts, it spreads across the surface and then retracts. But where the hydrophilic arms are, the drop lingers. This creates the four lobes we see on the droplet, and the asymmetric retraction gives the drop angular momentum. As it leaves the surface, the spin continues. In some configurations, the researchers could make the drop spin at more than 7300 rpm. (Image and research credit: H. Li et al; via Science; submitted by Kam-Yung Soh)

Avoiding Ice
Keeping ice from forming on a surface is a major engineering challenge. Typically, there’s no controlling certain factors – like the size and impact speed of droplets – so engineers try to tame ice by changing the surface. This can be through chemicals – as with deicing fluids used on aircraft – or by tuning the surface itself.
One way to do this is by making the surface superhydrophobic – or extremely water repellent. These surfaces are rough on a nanoscale level, but they’re delicate, and once ice gets a grip on them, it’s even harder to remove. In a recent study, however, researchers used particles with both hydrophobic and hydrophilic – water-attracting – properties to create a superior ice-resistant surface. The combination of hydrophobic and hydrophilic aspects to the particles made supercooled droplets break up on contact with the surface. This made the drops smaller and decreased their contact time, making it harder for them to stick and freeze. (Image credit: Pixabay; research credit: M. Schwarzer et al.; via Chembites; submitted by Kam-Yung Soh)

Entrained
When an object hits water whether it draws air in with it depends on its shape, impact speed, and surface characteristics. In this experiment, though, there’s a bit of a twist. Here the sphere is passing through an interface with surfactants added. On the left, the sphere passes through smoothly without entraining air or creating a cavity. On the right, the same sphere impacts at the same speed but this time the interface is covered in a layer of bubbles. As a result, the sphere pulls a large air cavity into the water with it. Why the big difference?
As the sphere passes through the bubbles, they burst, spraying the sphere with droplets. On impact, those droplets disrupt the layer of water traveling up the sides of the sphere, causing it to pull away from the surface and form a splash. Instead of smoothly coating the sphere in water, air can now stick to the sphere and get pulled in with it. (Image and research credit: N. Speirs et al., source)

Corrugating Water
The characteristics of a surface can have a major impact on the form a flow takes. The photo above shows a corrugated, almost pinecone-like water surface. It’s the result of a sheet of water flowing over a surface with alternating bands of hydrophobic (water-repelling) and hydrophilic (water-loving) properties. The water sheet narrows over hydrophobic sections and expands over hydrophilic ones. Gravity, inertia, and surface tension compete to create the overall braided appearance. You can see a top-down view of the flow in the original poster. (Image credit: M. Grivel et al., source)