2020 was certainly a strange year, and I confess that I mostly want to congratulate all of us for making it through and then look forward to a better, happier, healthier 2021. But for tradition and posterity’s sake, here were your top FYFD posts of 2020:
- Juvenile catfish collectively convect for protection
- Gliding birds get extra lift from their tails
- How well do masks work?
- Droplets dig into hot powder
- Updating undergraduate heat transfer
- Branching light in soap bubbles
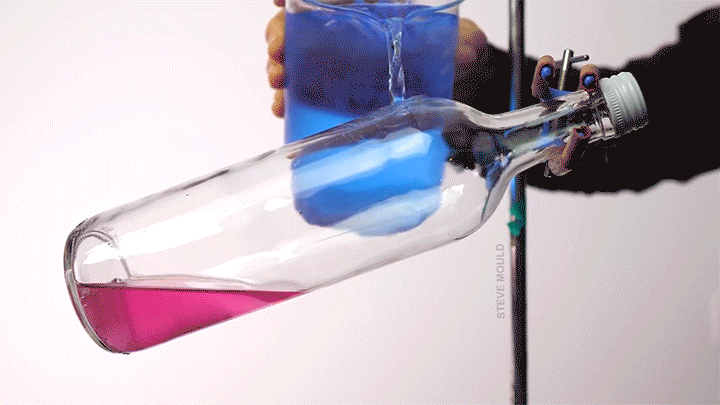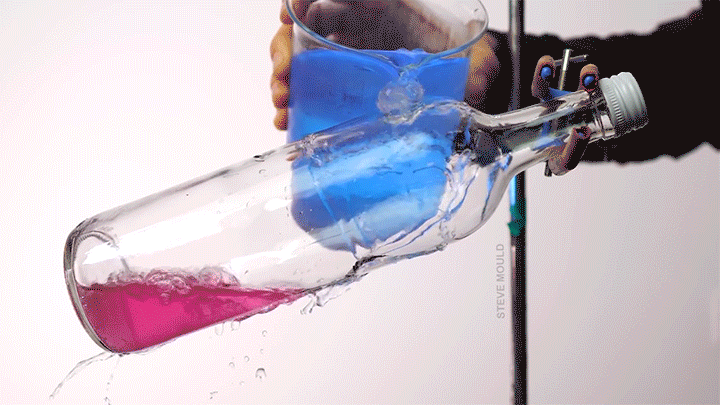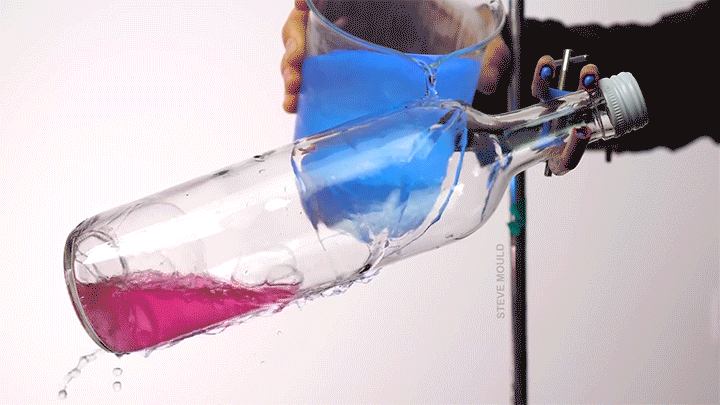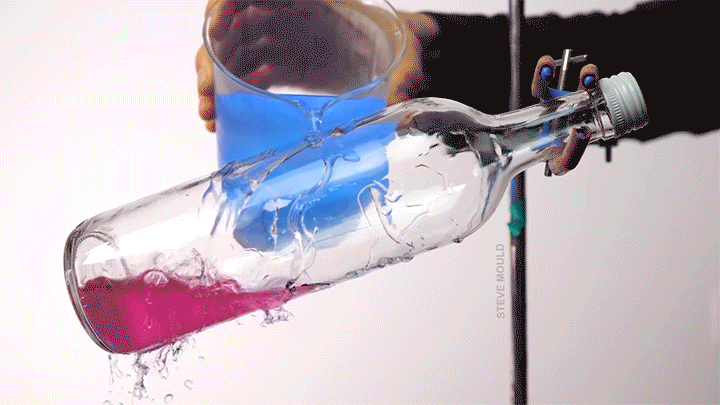
- Boiling water using ice water
- Concentric patterns on freezing and thawing ice
- Bouncing off superhydrophobic defects
- To beat surface tension, tadpoles blow bubbles
There’s a good mix of topics here! A little bit of biophysics, some research, some phenomena, and some good, old-fashioned fluid dynamics.
If you enjoy FYFD, please remember that it’s primarily reader-supported. You can help support the site by becoming a patron, making a one-time donation, buying some merch, or simply by sharing on social media. Happy New Year!
(Image credits: catfish – Abyss Dive Center, owl – J. Usherwood et al., masks – It’s Okay to Be Smart, droplet – C. Kalelkar and H. Sai, boundary layer – J. Lienhard, bubble – A. Patsyk et al., boiling – S. Mould, ice – D. Spitzer, defects – The Lutetium Project, tadpoles – K. Schwenk and J. Phillips)