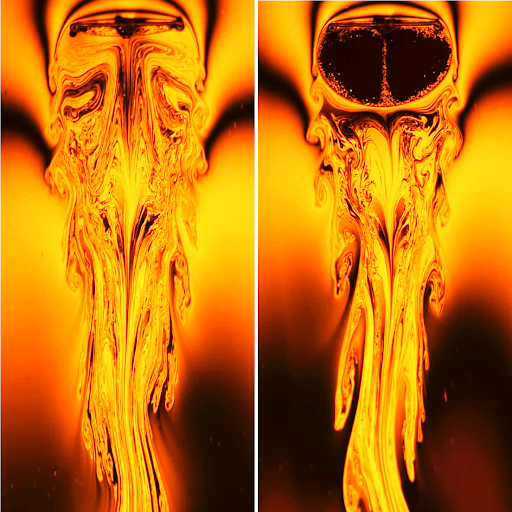
Macro images of a soap film burst with color. Because the color comes from interference between light waves bouncing off the inner and outer surfaces of the soap film, the colors we see correspond directly to the thickness of the soap film. So the patterns we see reflect actual flows and variations inside the soap film. It’s not unusual for the patterning on a soap film to become increasingly complicated as the film drains and ages. Eventually black spots — areas too thin for interference to show visible colors — will appear and grow, and the film will pop.
If you’re interested in trying out some soap film photography for yourself, Professor Andrew Davidhazy has a nice description on his website of the set-up he used for this photo. (Image credit: A. Davidhazy; via Flow Vis)