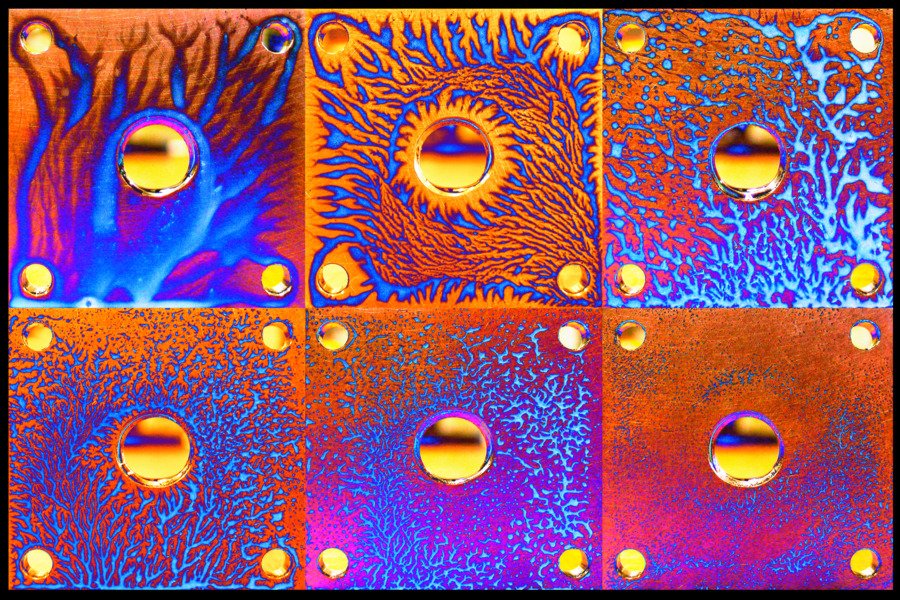
Fluid phenomena can show up in unexpected places. The collage above shows patterns formed when an aluminum block is lifted during wet sanding, a polishing technique. The dendritic fingers are formed from oil and the slurry of sanded particles being polished away. They are an example of the Saffman-Taylor instability, which forms when less viscous fluids (oil) protrude into a more viscous one (the slurry). Each image contains a different concentration of oil, resulting in very different fingering patterns. (Image credit: D. Lopez)
Tag: Saffman-Taylor instability

Viscous Fingers
Viscous fingers form between air and titanium dioxide sol-gel in this photograph. The two fluids are trapped in a thin gap between glass plates – a set-up known as a Hele-Shaw cell. The dendritic fingers we see form when the less viscous air pushes into the more viscous sol-gel. This is an example of the Saffman-Taylor instability. The psychedelic colors are a result of thin-film interference and the way light interacts with very thin materials. The same effect is responsible for the colors on soap bubbles. (Image credit: C. Trease)

Viscous Fingers
Take a viscous fluid, like laundry detergent, and sandwich it between two plates of glass. Fluid dynamicists call this set-up a Hele-Shaw cell. If you then inject a less viscous fluid, like air, between the plates–or if you try to pry them apart–you’ll see a distinctive pattern of dendritic fingers form. This viscous fingering, also known as the Saffman-Taylor instability, occurs because the interface between the two fluids is unstable. Invert the problem, though–inject a more viscous fluid into a less viscous one–and no special shapes will form because the interface will remain stable. (Image credit: Random Walk Studios, source)

Viscous Fingers
Viscous liquid placed between two plates forms a finger-like instability when the top plate is lifted. The photos above show the evolution of the instability for four initial cases (top row, each column) in which the initial gap between the plates differs. Each row shows a subsequent time during the lifting process. As the plate is pulled up, the viscous liquid adheres to it and air from the surroundings is entrained inward to replace the fluid. This forms patterns similar to the classic Saffman-Taylor instability caused when less viscous fluid is injected into a more viscous one. (Photo credit: J. Nase et al.)

Elastic Walls and Viscous Fingers
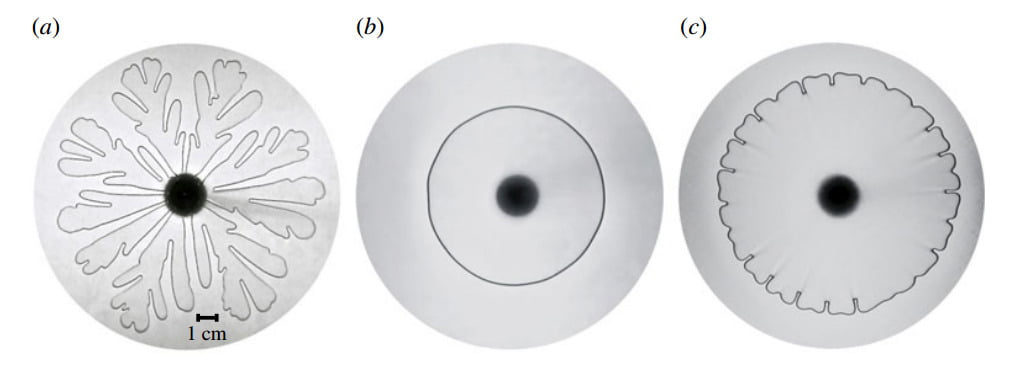
The Saffman-Taylor instability, characterized by the branchlike fingers formed when a less viscous fluid is injected into a more viscous one, is typically demonstrated between two rigid walls, as in part (a) of the figure above. But what happens if one of the rigid walls forming the Hele-Shaw cell is replaced with an elastic wall? This is the case for (b) and (c) in the figure. The flexibility of the wall causes the expansion of the air-fluid interface to slow down relative to the rigid wall case and causes the interface to move toward a narrowing fluid-filled gap (as opposed to a constant thickness one). Both of these effects reduce the viscous instability mechanism that drives the fingering instability. With a high enough mass flow rate as in ©, there is still some instability in the interface, but it is dramatically reduced. (Photo credit: D. Pihler-Puzovic et al.)

Protruding Fingers
Instability is a common feature of fluid flows and can generate a near infinite set of patterns. The video above shows the Saffman-Taylor instability, an interface instability that occurs when a fluid of lower viscosity is injected into a higher viscosity fluid. In this case, the fluids inhabit a thin space between two glass plates. The less viscous fluid displaces the more viscous one in a series of branching finger-like shapes. If the situation were reversed, with a more viscous fluid injected into a less viscous one, the interface would be stable and expand radially without any pattern formation. (Video credit: William Jewell College)

Viscous Fingers
High viscosity silicon oil is sandwiched between two circular plates. As the upper plate is lifted at a constant speed, air flows in from the sides. The initially circular interface develops finger-like instabilities, due to the Saffman-Taylor mechanism, as the air penetrates. Eventually the fluid will completely detach from one plate. (Photo credit: D. Derks, M. Shelley, A. Lindner)

Viscoelastic Fingers
This series of photos shows two plates with a thin layer of polymer-laced, viscoelastic liquid. As the two plates are separated, complex instabilities form. The lower section of each photograph shows the fluid on the plate, with finger-like Saffman-Taylor instabilities forming as air rushes in between the gap in the plates. As the separation increases, the polymers in the liquid stretch under the increased strain, inducing elastic stresses in the fluid that cause the formation of secondary structures. (Photo credit: R. Welsh, J. Bico, and G. McKinley)

Viscous Fingers
When less viscous fluids are injected into a more viscous medium, the low-viscosity fluid forms finger-like protrusions into the background fluid. This is known as the Saffman-Taylor instability. The video above shows this effect but in a more dynamic setting. Blue-dyed water and a clear solution of water and glycerol fifty times more viscous than the water are injected in alternating fashion to a microfluidic channel. The blue water spreads into the clear glycerol solution via fingers that quickly diffuse, creating a homogeneous–or uniform–mixture. (Video credit: Juanes Research Group)

Squeezing Bubbles
An air bubble trapped inside a viscoelastic fluid is squeezed between two plates in this video, revealing a Saffman-Taylor-like fingering instability stemming from local stress concentrations. (Video credit: Baudouin Saintyves)