The Slow Mo Guys bring their high-speed skills to underwater explosions in this new video. The physics of such explosions is very neat (but also incredibly destructive). When the fuse ignites, a blast wave travels outward in a sphere, creating a bubble filled with gas. Eventually, the pressure of the surrounding water is too great for the bubble to expand against. When its expansion slows, that much larger pressure from the surrounding water starts to crush the bubble back down. Decreasing the volume of the bubble raises its pressure and its temperature again, and this often reignites any leftover fuel and oxidizer left in the bubble. The secondary shock bubble will re-expand, kicking off another round of expansion and collapse. (Video credit: The Slow Mo Guys; submitted by potato-with-a-moustache)
Tag: explosion

Quarry Smashing
Despite appearances, this is not a crashing ocean wave. In fact, it’s a planned explosion at a quarry, and that wave is more than 360,000 tons of rock and 68 tons of explosive pouring down. The scale of this is hard to imagine, and the physics of a ocean breaker and a massive wave of rocks and gas are similar enough that it’s no wonder our brains interpret them as the same event. Visual effects artists have been using this trick for decades. Rather than simulate the motion of a true fluid, many CGI effects are created from digital particles that, much like the rocks above, are similar enough to fool our eyes and our brains. (Image credit: K. Venøy, source; via Gizmodo)

When Lasers Strike
Lasers are a great way to deliver a lot of energy very quickly. In this animation, you see a jet of water get struck by a pulse from a powerful X-ray laser. The energy from that laser pulse gets absorbed by the water in a matter of picoseconds – that’s trillionths of a second. All that energy in so little time makes the water vaporize explosively. It’s this vapor explosion that breaks the jet in two. As the vapor expands outward, it forces water from the jet into a thin film that forms a cone. The conical film bends back on itself until it strikes the jet and coalesces. For more, check out this video of a similar experiment that looked at laser impacts on droplets. (Image credit: C. Stan et al., from Supplementary Movie 5; via Gizmodo)

Underwater Explosions
Underwater explosions are incredibly dangerous and destructive, and this animation shows you why. What you see here are three balloons, each half-filled with water and half with air. A small explosive has been set off next to them in a pool. In air, the immense energy of an explosion actually doesn’t propagate all that far because much of it gets expended in compressing the air. Water, on the other hand, is incompressible, so that explosive energy just keeps propagating. For squishy, partially air-filled things like us humans or these balloons, that explosion’s force transmits into us with nearly its full effect, causing expansion and contraction of anything compressible inside us as our interior and exterior pressures try to equalize. The results can be devastating. To see the equivalent experiment in air, check out Mark Rober’s full video on how to survive a grenade blast. (Image credit: M. Rober, source)

Molten Salt in Water
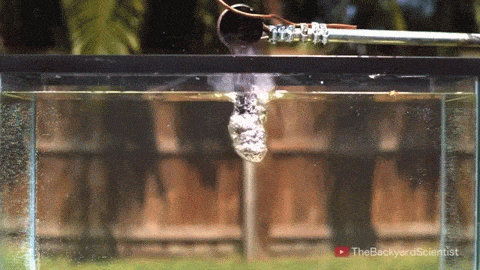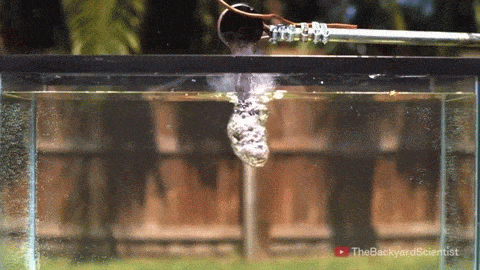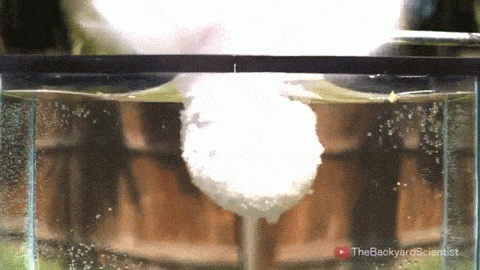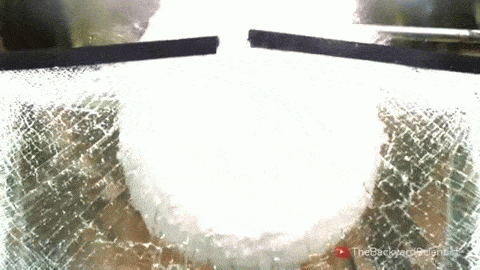
In his latest video, The Backyard Scientist explores what happens when molten salt (sodium chloride) gets poured into water. As you can see, the results are quite dramatic! He demonstrates pretty convincingly that the effect is physical – not chemical. The extreme difference in temperature between the liquid water (< 100 degrees Celsius) and the molten salt (> 800 degrees Celsius) causes the water to instantly vaporize due to the Leidenfrost effect. This vapor layer protects the liquid water from the molten salt – until it doesn’t. When some driving force causes a drop of water to touch the salt without that protective vapor layer, the extreme temperature difference superheats the water, causing it to expand violently, which drives more water into salt and feeds the explosion.
But why don’t the other molten salts he tests explode? Sodium carbonate, the third salt he tests, has a melting point of 851 degrees Celsius, 50 degrees hotter than sodium chloride. Yet for that test, the Leidenfrost effect prevents any contact between the two liquids. The key in this case, I hypothesize, is not simply the temperature difference between the water and salt, but the difference in fluid properties between sodium chloride and sodium carbonate. The breakdown of the vapor layer and subsequent contact between the water and the molten salt depends in part on instabilities in the fluids. A cavity where instabilities can grow more easily is one where the Leidenfrost effect is less likely to protect and separate the two fluids. And, in fact, it turns out that the surface tension of molten sodium chloride is significantly lower than that of molten sodium carbonate! A lower surface tension value means that the molten sodium chloride breaks into droplets more easily and its vapor cavity will respond more strongly to fluid instabilities, making it more likely to come in contact with liquid water and, thus, cause explosions. (Image/video credit: The Backyard Scientist; submitted by Simon H)

Seeing Blast Waves
With a large enough explosion, it’s actually possible to see shock waves. This high-speed camera footage shows the detonation of a car packed with explosives. After the initial flash, you can see the thin membrane of the blast wave expanding outward. This shock wave is a traveling discontinuity in the air’s properties–temperature, pressure, and density all change suddenly over an incredibly small distance. It’s this last variable–density–that enables us to see the effect. Density has a significant impact on air’s index of refraction (which also explains heat mirages). In this case, the shift in refractive index is large enough that we see the difference relative to the background, enabling our eyes to follow an otherwise invisible effect. (Video credit: Mythbusters/Discovery Channel; via Gizmodo)
If you enjoy FYFD, please help support me by becoming a patron!

Underwater Explosions
As dangerous as explosions are in air, they are even more destructive in water. Because air is a compressible fluid, some part of an explosion’s energy is directed into air compression. Water, on the other hand, is incompressible, which makes it an excellent conductor of shock waves. In the video above we see some simple underwater explosions using water bottles filled with dry ice or liquid nitrogen. The explosions pulsate after detonation due to the interplay between the expanding gases and the surrounding water. When the gases expand too quickly, the water pressure is able to compress the gases back down. When the water pushes too far, the gases re-expand and the cycle repeats until the explosion’s energy is expended. This pulsating change in pressure is part of what makes underwater explosions so dangerous, especially to humans. Note in the video how the balloons ripple and distort due to the changing pressure. Those same changes in pressure can cause major internal damage to people. (Video credit: The Backyard Scientist; submitted by logicalamaze)

Blast Waves Visualized
Typically, shock waves are invisible to the human eye. Using sensitive optical techniques like schlieren photography, researchers in a lab can visualize sharp density gradients like shock waves or even the slight density variations caused by natural convection. But it takes some special conditions to make shock waves visible to the naked eye. The blast wave of the explosion in the photo above is a great example. The leading edge of the shock wave and the heat of the explosion create a strong, sharp change in density. That density change is accompanied by a change in the air’s refractive index. As light travels from the distance toward the camera, it’s distorted–more specifically, refracted–when it travels through the blast wave and its wake. And, in this case, that visual distortion is strong enough that we can clearly see the outlines of the shock waves moving out from the explosion. The apparent horizontal line through the blast wave is probably the intersection of a weaker secondary shock wave with the initial expanding shock wave. (Image credit: Defense Research and Development Canada; via io9)

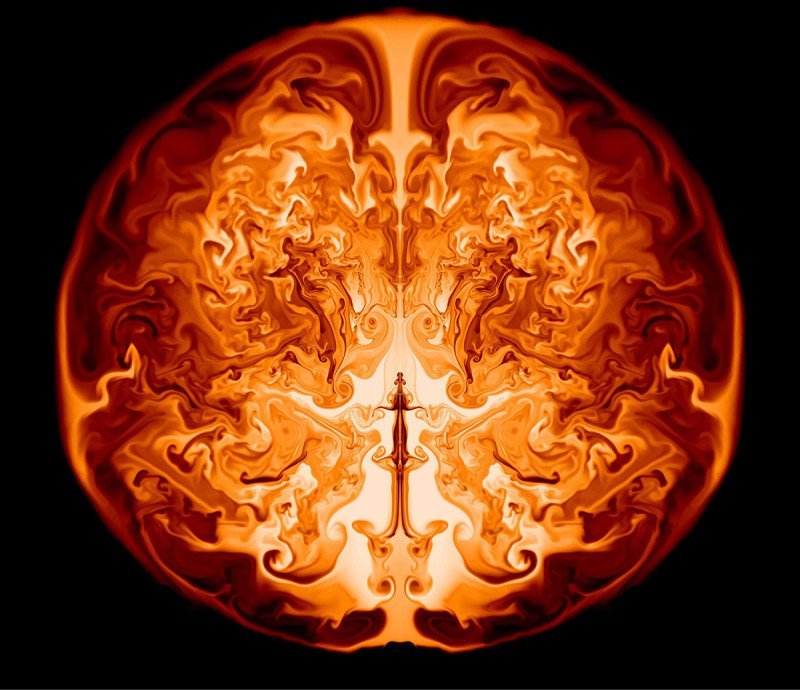
Supernova Simulation
New research shows that supermassive first-generation stars may explode in supernovae without leaving behind remnants like black holes. The work is a result of modeling the life and death of stars 55,000 to 56,000 times more massive than our sun. When such stars reach the end of their lives, they become unstable due to relativistic effects and begin to collapse inward. The collapse reinvigorates fusion inside the star and it begins to rapidly fuse heavier elements like oxygen, magnesium, or even iron from the helium in its core. Eventually, the energy released overcomes the binding energy of the star and it explodes outward as a supernova. The image above is a slice through such a star approximately one day after its collapse is reversed. Hydrodynamic instabilities like the Rayleigh-Taylor instability produce mixing of the heavy elements throughout the expanding interior of the star. The mixing should produce a signature that can be observed in the aftermath as these stars seed their galaxies with the heavy elements needed to form planets. For more, see Science Daily and Chen et al. (Image credit: K. Chen et al., via Science Daily; submitted by mechanicoolest)

Krakatoa
Volcanoes seem to be a common topic these days. Yesterday Nautilus published a great piece by Aatish Bhatia on the 1883 eruption of Krakatoa, which tore the island apart and unleashed a sound so loud it was heard more than 4800 km away:
The British ship Norham Castle was 40 miles from Krakatoa at the time of the explosion. The ship’s captain wrote in his log, “So violent are the explosions that the ear-drums of over half my crew have been shattered. My last thoughts are with my dear wife. I am convinced that the Day of Judgement has come.“
In general, sounds are caused not by the end of the world but by fluctuations in air pressure. A barometer at the Batavia gasworks (100 miles away from Krakatoa) registered the ensuing spike in pressure at over 2.5 inches of mercury. That converts to over 172 decibels of sound pressure, an unimaginably loud noise. To put that in context, if you were operating a jackhammer you’d be subject to about 100 decibels. The human threshold for pain is near 130 decibels, and if you had the misfortune of standing next to a jet engine, you’d experience a 150 decibel sound. (A 10 decibel increase is perceived by people as sounding roughly twice as loud.) The Krakatoa explosion registered 172 decibels at 100 miles from the source. This is so astonishingly loud, that it’s inching up against the limits of what we mean by “sound.” #
Those are some mindbogglingly enormous numbers. Aatish does a wonderful job of explaining the science behind an explosion whose effects ricocheted through the atmosphere for days afterward. Check out the full article over at Nautilus. (Image credit: Parker & Coward, via Wikipedia)