Sometimes a droplet needs a little protection while it’s traveling to its destination. When that’s the case, we often try to encapsulate it in a layer of material that won’t be affected by whatever environment the drop is traveling through. In this study, researchers aimed to give their drops not one but two layers of protection — in as simple a way as possible.
The team began with three layers of liquid. The lowest layer was water, the middle layer was an oil, and the top layer was a mixture of water and isopropyl alcohol. Next, they added glass particles that were denser than the alcohol, but less dense than the oil. This caused the particles to form a clump — a granular raft — along the interface between the alcohol and the oil (not shown). When the layer of particles became heavy enough, it began to sink into the oil, carrying some of the alcohol with them. This conglomeration formed the initial droplet of alcohol mixture encased in an armor of glass beads.
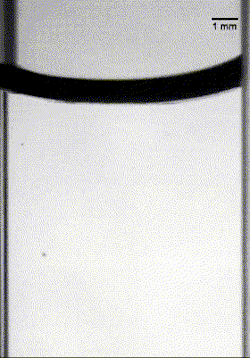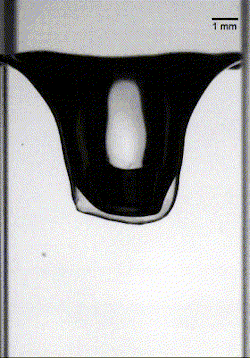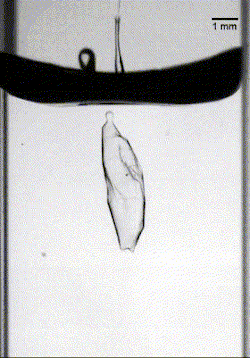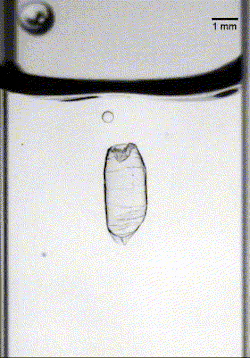
As this armored droplet sank, it approached the second interface: the oil-water interface. At this juncture, the team observed three different outcomes. When the glass particles were small or light, the armored drop would come to a rest at the oil-water interface. As the drop deformed, water would pierce the armor, causing the whole drop to rupture (Image 1).
In the second case, heavier particles caused the armored drop to sink through the oil-water interface, but a low oil viscosity meant that the oil film drained from the bottom of the drop before the drop was fully encapsulated. Once again, this let the water through and ruptured the droplet (Image 2).
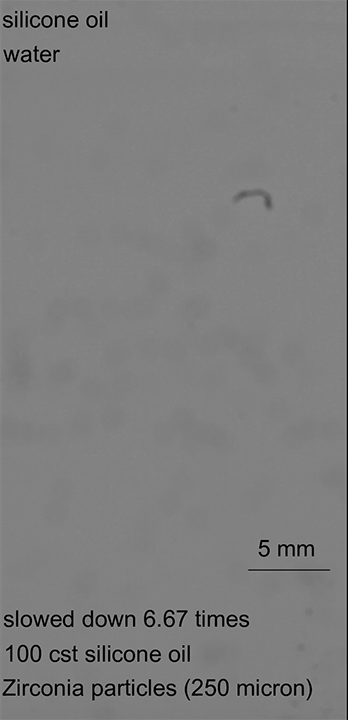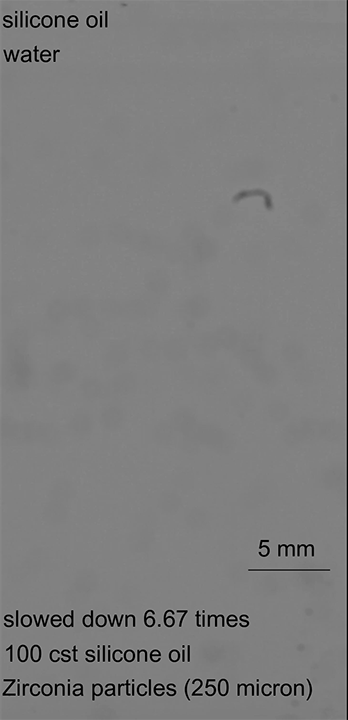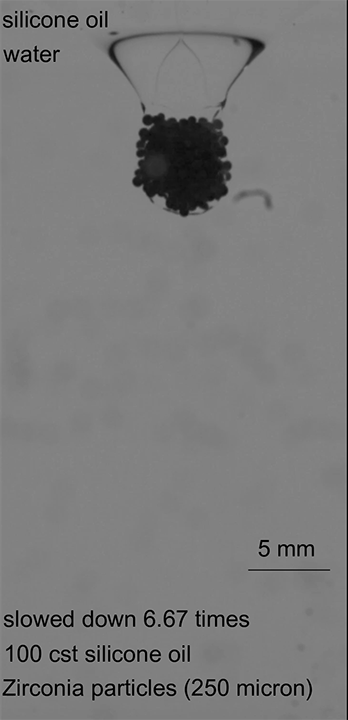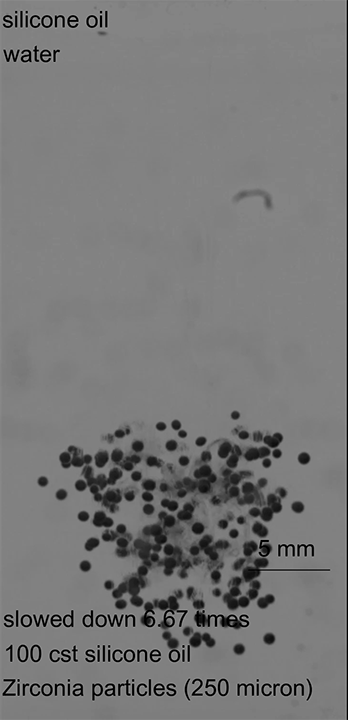
In the final case, armored drops with just the right bead density and oil viscosity would sink through the oil-water interface until the oil pinched off behind the drop. This pinch-off allowed the oil to redistribute around the drop, encapsulating it in layers of both oil and particles, thereby protecting it as it continued its journey (Image 3). (Image credits: top – Girl with red hat, experiment – A. Hooshanginejad et al.; research credit: A. Hooshanginejad et al.)