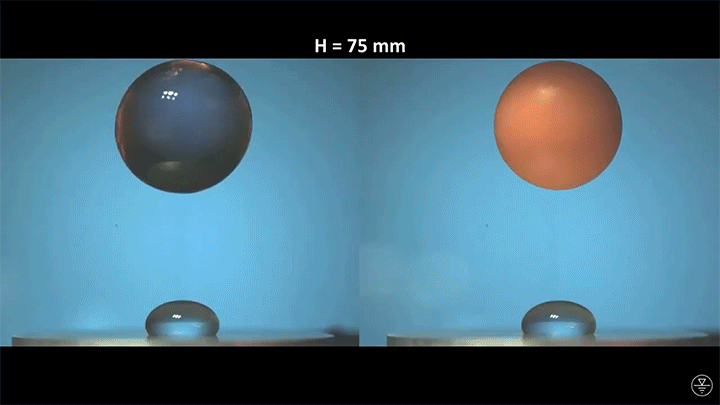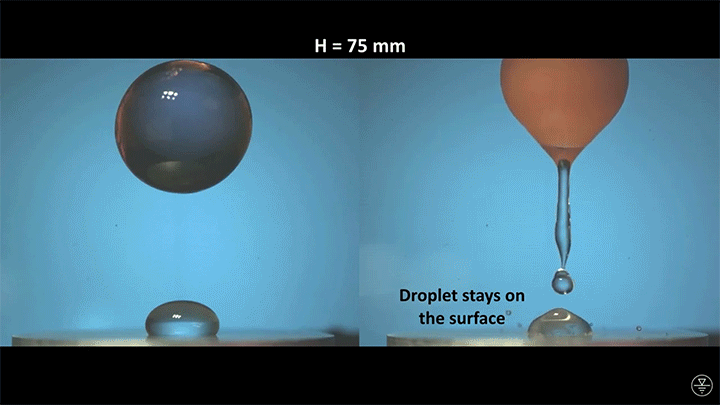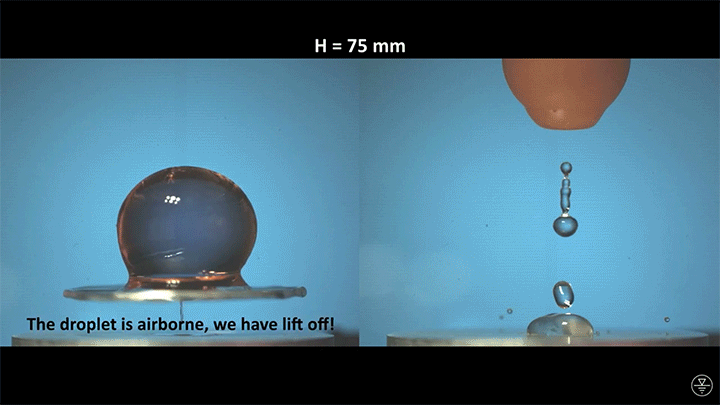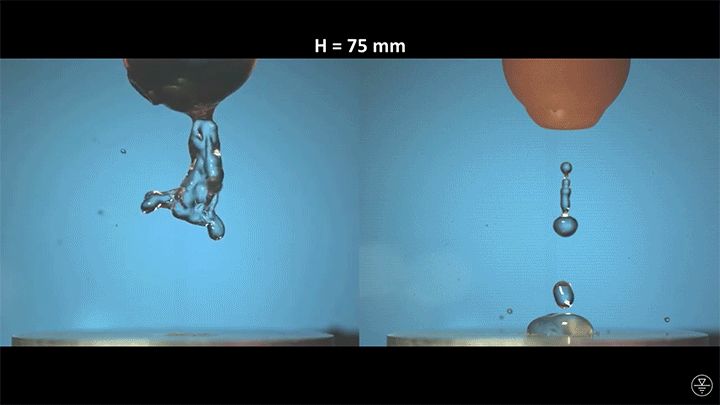
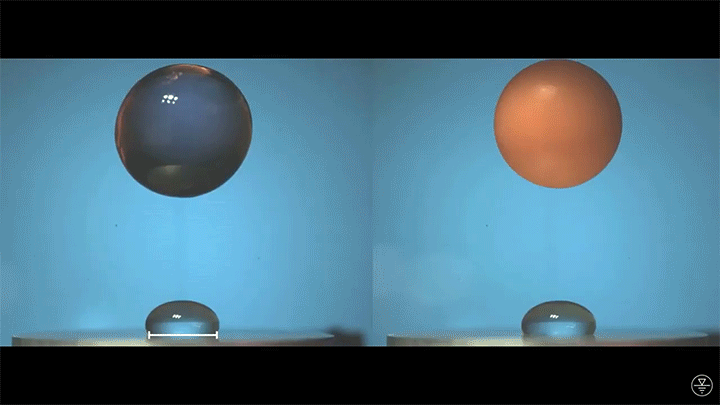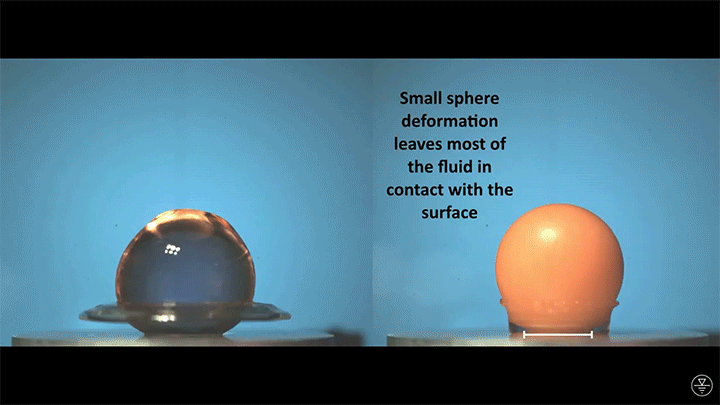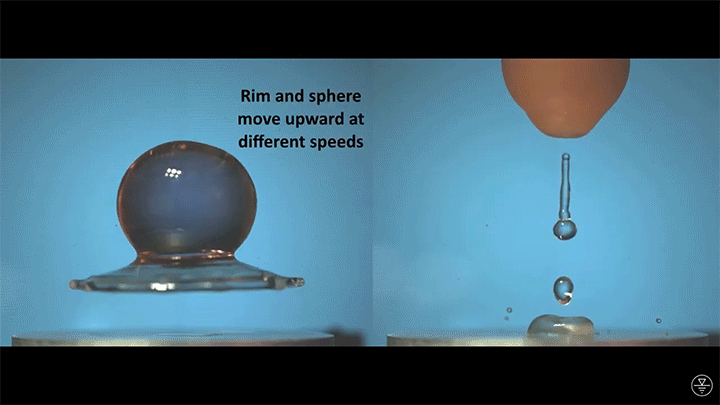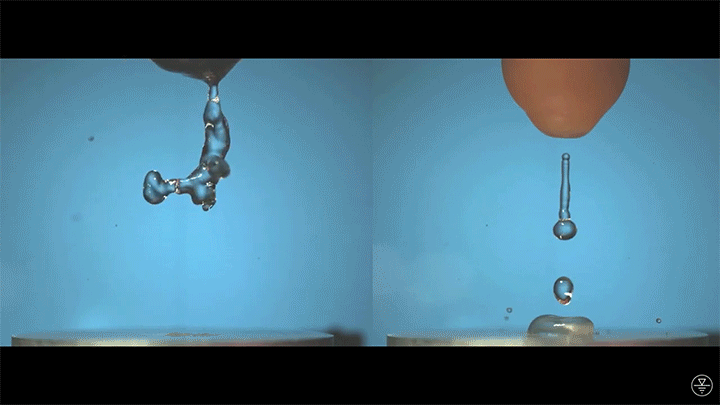
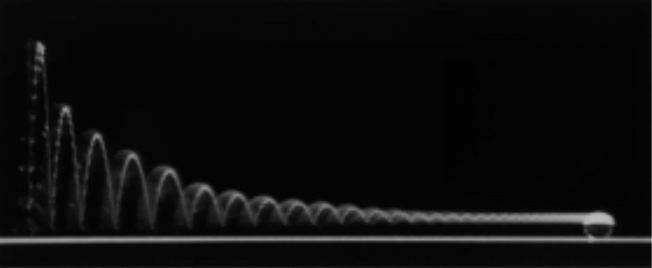
A droplet falling on a liquid bath may, if slow enough, rebound off the surface. Its impact sends out a string of ripples — capillary waves — on the bath’s surface and sends the droplet itself into jiggling paroxysms. A new pre-print study delves into this process through a combination of experiment, simulation, and modeling. Impressively, they find that the most of the droplet’s initial energy is not dissipated during impact. Instead it’s fed into the capillary waves and droplet deformation that follow. (Image and research credit: L. Alventosa et al.; via Dan H.)