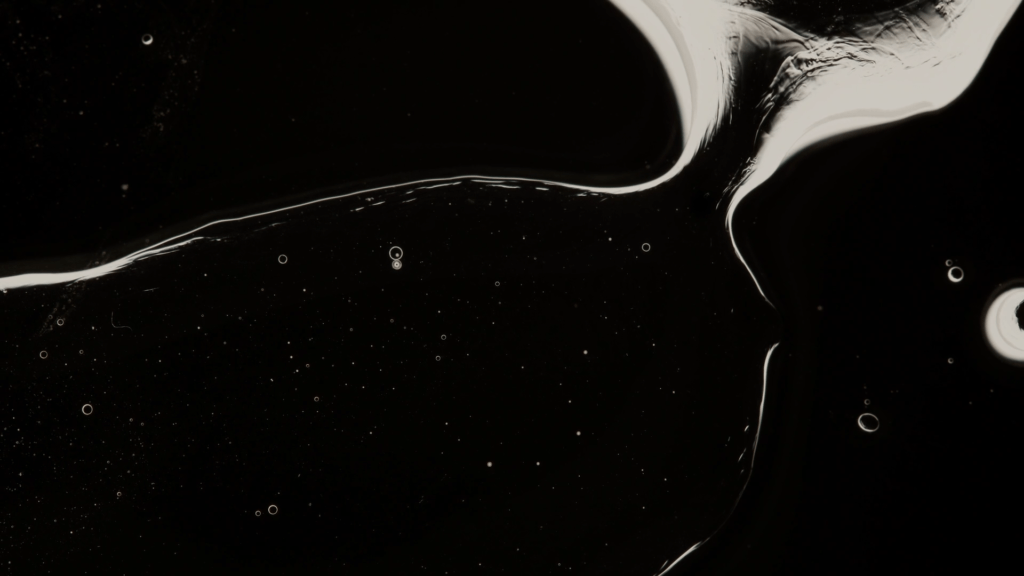
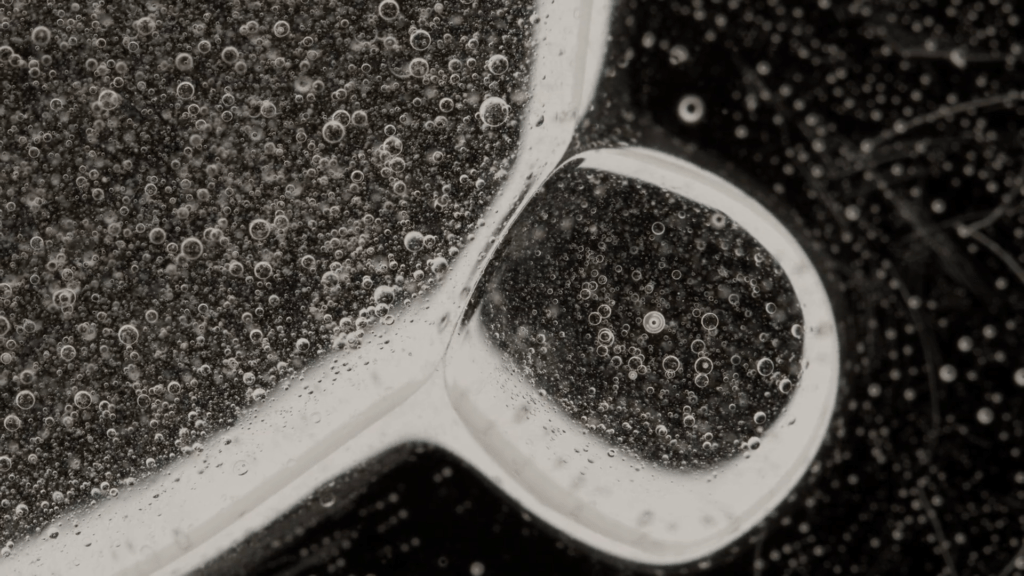
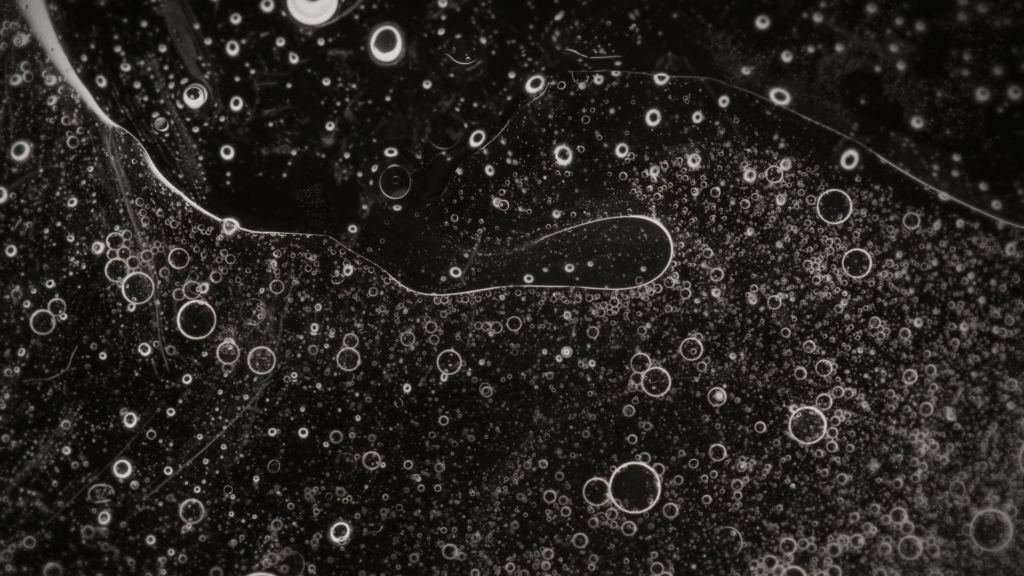
A blob of sunflower oil floating on soapy water forms a disk known as a liquid lens. But add some dyed ethanol and things take a turn. The lens rapidly expands and distorts as the ethanol and soapy water meet. These surface flows are driven by the imbalance of surface tension between the different liquids. The liquid lens deforms and abruptly ruptures, releasing dye and ethanol before rebounding into a stable lens again. Adding more ethanol to the lens will repeat the cycle. (Image credit: C. Kalelkar and P. Dey; research credit: D. Maity et al.)
Tag: marangoni effect

Long-Lived Bubbles
Without surfactants to stabilize them, bubbles don’t last long at room temperature. But adding a little heat changes the picture. When heated, the bubbles get stabilized by a thermal gradient that lifts fluid toward the bubble’s peak, where it cools and gathers. Eventually, the cold fluid grows heavy enough to sink down the side of the bubble (in either a constant stream or occasional drips); with warm fluid getting pulled up to replace it (via the Marangoni effect), the process repeats and the bubble lives on. (Video credit: S. Nath et al.; see also)

Surfactants and Waves
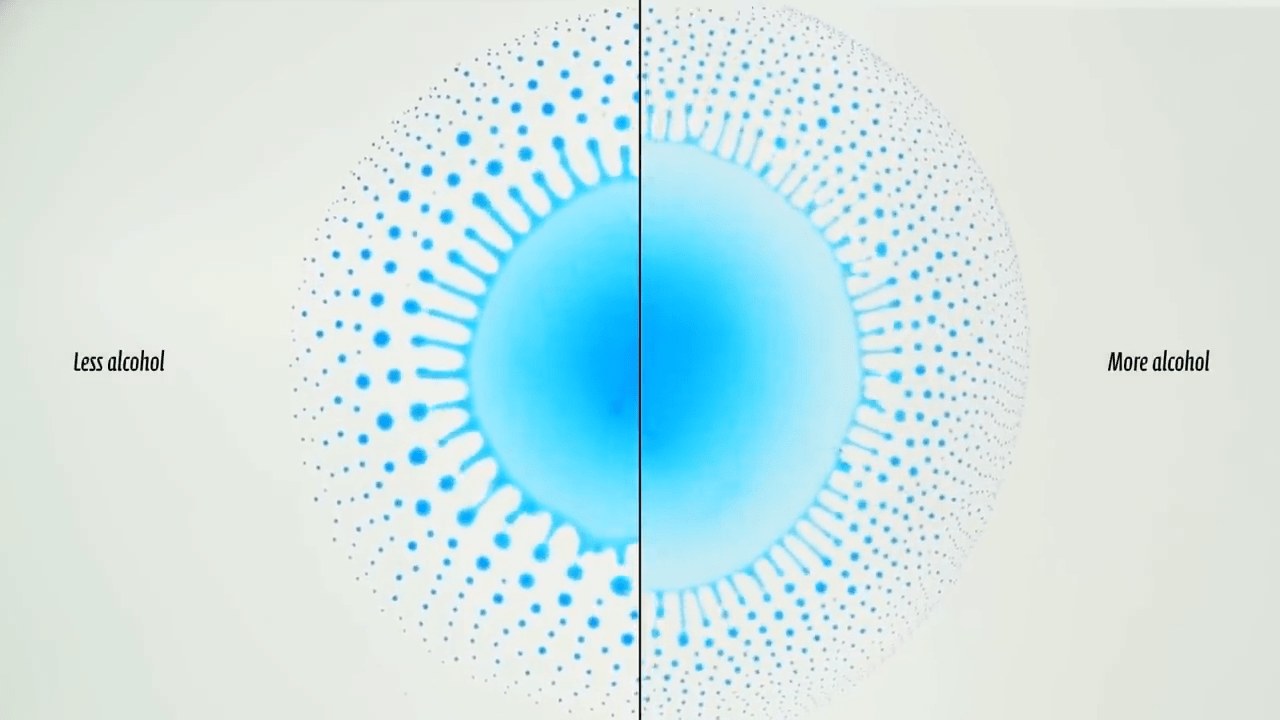
In the ocean, waves often curl over and trap air, becoming plunging breakers. How do surfactants like soap or oil affect this process? That’s the question behind this video, where researchers visualize breaking waves with differing amounts of added surfactant. In the case of pure water, the wave forms a smooth jet that curls over and traps air when the wave breaks. As more and more surfactant gets added, the shape of that jet and cavity change. In one case, they become irregular. In another, they disappear entirely, and with the most surfactant added, the wave suddenly looks just like the water-only case.
The key to these behaviors, it turns out, is not how much surfactant there is, but how much the concentration of surfactant varies along the length of the wave. When there are significant changes in the surfactant concentration along the wave, local Marangoni flows try to even out the surface tension, causing the wave to break up in an irregular fashion. (Image and video credit: M. Erinin et al.)

Self-Propelled Droplets
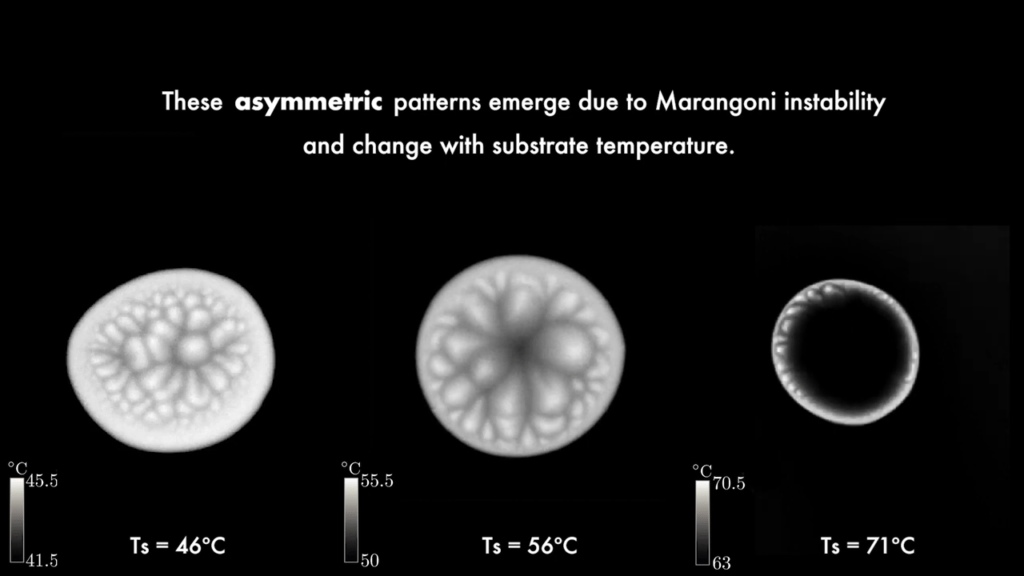
Drops of ethanol on a heated surface contract and self-propel as they evaporate. My first thought upon seeing this was of Leidenfrost drops, but the surface is not nearly hot enough for that effect. Instead, it’s significantly below ethanol’s boiling point. Looking at the drops in infrared reveals beautiful, shifting patterns of convection cells on the drop. The patterns are driven by the temperature difference along the drop; at the bottom, the drop is warmest, and at its apex, it is coldest. Those differences in temperature create differences in surface tension, which drives a surface flow that breaks the drop’s symmetry. The asymmetry, the authors suggest, is responsible for the drop’s propulsion. (Image and video credit: N. Kim et al.)

Soapy Solutions
When a drop of soap falls into a pool of water, its surface-loving molecules spread out on the water’s surface. Exactly how the soap spreads depends on the local concentration of its surfactant molecules, which create areas with different surface tensions that cause flow. All in all, it’s a tough process to predict because it varies in time at every point on the pool. But a recent paper offers a new class of exact solutions for the problem.
The paper considers a surfactant-laden droplet spreading over a (relatively speaking) deep pool. Other researchers showed recently that this situation can be described with a complex version of the Burgers’s equation, which was originally developed to describe turbulent flows. The authors solved the equation for a variety of initial conditions and found that the time-dependent spread of the surfactants was sensitive to the initial surface distribution. The higher the initial surface concentration, the faster the surfactants spread. (Image credit: T. Despeyroux; research credit: T. Bickel and F. Detcheverry; via APS Physics; submitted by Kam-Yung Soh)

“Titan”
Saturn’s moon Titan is a fascinating foil to our planet. It’s the only other body in our solar system with liquid bodies — lakes and seas — on its surface. But where Earth’s oceans are filled with water, Titan’s frigid lakes are liquid hydrocarbons. This video, “Titan,” is a short film inspired by the moon’s seas and is made up of various liquids and chemical reactions filmed under magnification. Sit back and enjoy the flow! (Image and video credit: S. Bocci/Julia Set Lab)

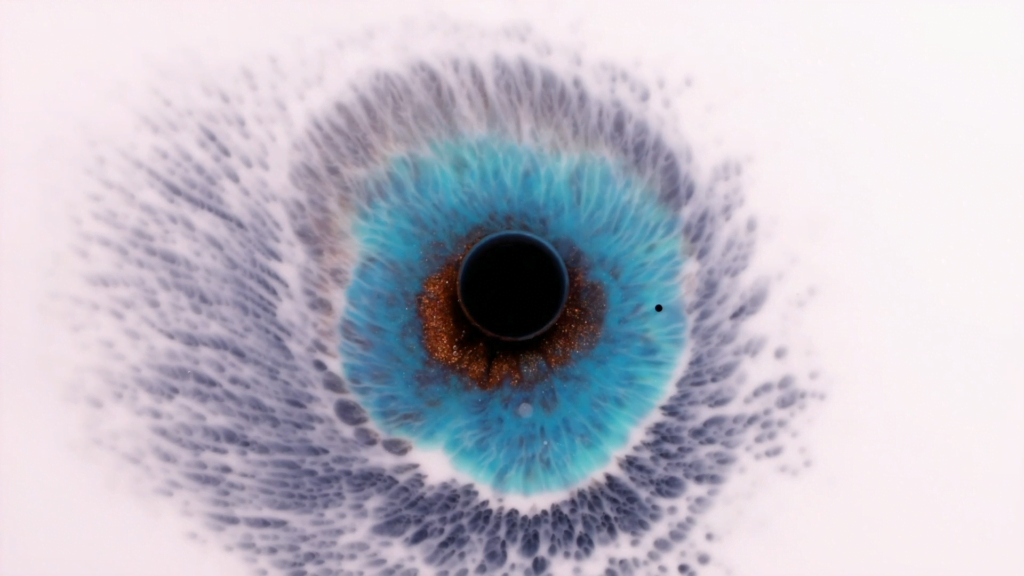
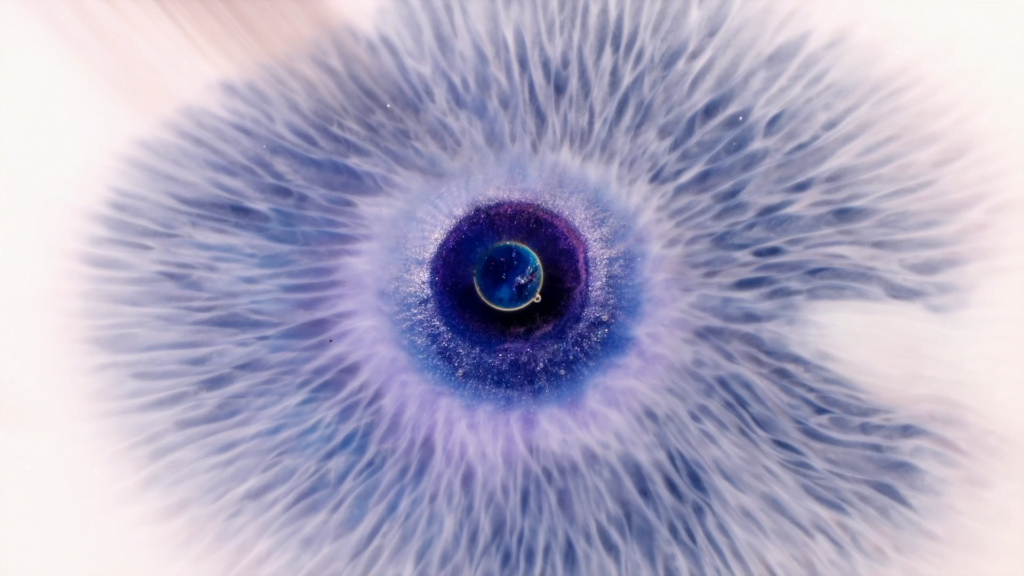
“I See You”
In “I See You,” filmmaker Rus Khasanov captures fluid flows that give the screen an eye with which to gaze back at us. The textures visible in the flows are incredible at mimicking the details of a human iris. These are some seriously neat Marangoni flows. For a similar effect, check out this film of his. (Image and video credit: R. Khasanov)

When Seeing a Flow Changes It
Adding dye to a flow is a common technique for visualization. After all, many flows in fluids like air and water are invisible to our bare eyes. But for some classes of flows — especially those driven by variations in surface tension — adding dye can have unforeseen effects. A recent study shows how true this is for bursting Marangoni droplets, where evaporation and alcohol concentration can pull a water-alcohol droplet apart.

As more dye is added to the experiment, the daughter droplets grow larger and more ligaments form. In the first three images, a dashed black line has been added to show the location of the droplet rim. Without dye, it’s nearly impossible to see the phenomenon since the refractive indices of the two component liquids are so close. But the researchers found that, as they added more methyl blue dye, it did more than increase the contrast in the flow. It changed the flow, making the droplets larger and creating ligaments between them. They believe that the dye’s own surface tension creates local gradients that alter the flow. It’s a reminder that experimentalists have to be careful to consider how our efforts to measure and observe a flow can change it. (Image credit: top – The Lutetium Project, bottom – C. Seyfert and A. Marin with modification; research credit: C. Seyfert and A. Marin)

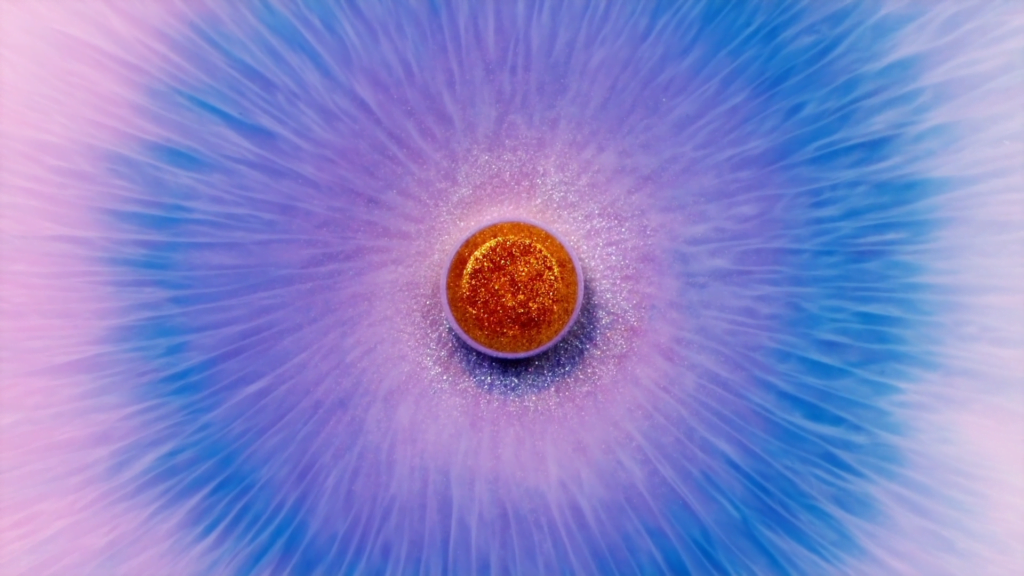
“Velocity”
In this short film by Vadim Sherbakov, macro shots of glittery ink and pigments look like astronomical vistas. The title of the film, “Velocity,” is spot on; every shot is full of flow and motion driven by the mixture of ink, alcohol, soap, and other fluids. That means lots of surface-tension-driven flow, and the glitter particles act as excellent tracers, giving a real sense of depth and direction for our gaze to follow. Watching films like this, I always want to pull out some odds and ends and try it for myself, but I’m certain my results would pale in comparison! (Video and image credit: V. Sherbakov; via Colossal)

“Heaven”
Wispy white cirrus clouds cover dark skies glittering with stars in Roman De Giuli’s “Heaven”. Or so it appears. In reality, these skyscapes are made with watercolors, ink, and acrylic paint. The vistas are gorgeous regardless of whether they’re driven by turbulent convection (as in the atmosphere) or the Marangoni effect (as in this video)! (Video and image credit: R. De Giuli)