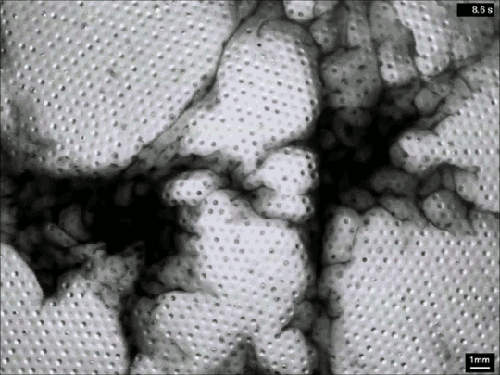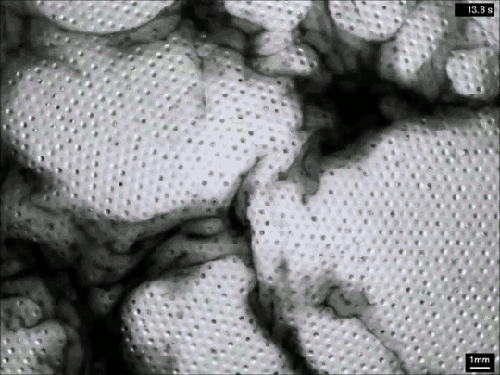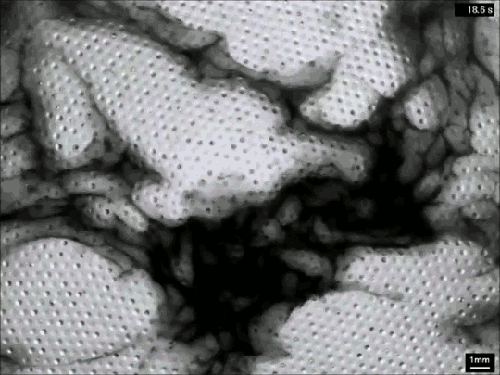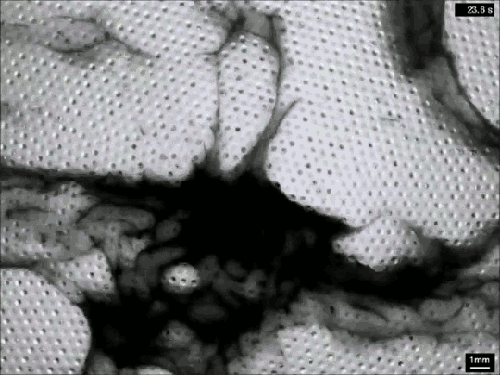
Despite their ubiquity and importance, we know surprisingly little about how clouds form. The broad strokes of the process are known, but the details remain somewhat fuzzy. One challenge is understanding how nucleation – the formation of droplets that become clouds or rain – works. A recent laboratory experiment in an analog cloud chamber suggests that falling rain drops may help spawn more rain drops.
The experiment takes place in a chamber filled with sulfur hexafluoride and helium. The former acts like water in our atmosphere, appearing in both liquid and vapor forms, while the latter takes the place of dry components of our atmosphere, like nitrogen. The bottom of the chamber is heated, forming a liquid layer of sulfur hexafluoride, seen at the bottom of the animation above. The top of the chamber is cooled, encouraging sulfur hexafluoride vapor to condense and form droplets that fall like rain. A top view of the same apparatus during a different experiment is shown in this previous post.
When droplets fall through the chamber, their wakes mix cold vapor from near the drop with warmer, ambient vapor. This changes the temperature and saturation conditions nearby and kicks off the formation of microdroplets. These are the cloud of tiny black dots seen above. Under the right conditions, these microdroplets grow swiftly as more vapor condenses onto them. In time, they grow heavy enough to fall as rain drops of their own. (Image credits: P. Prabhakaran et al.; via APS Physics; submitted by Kam-Yung Soh)