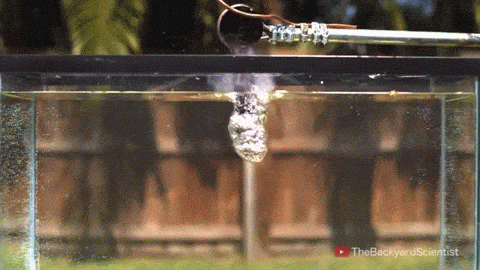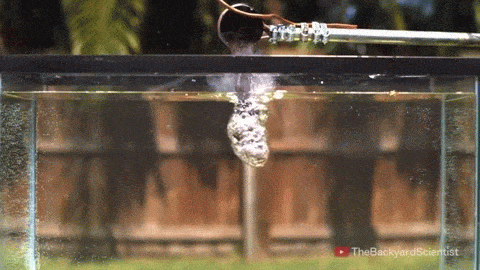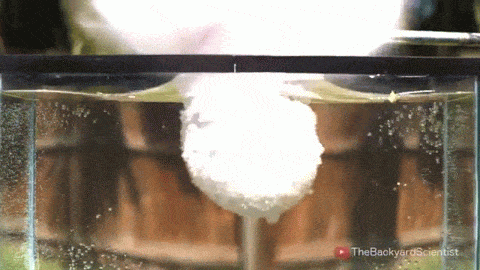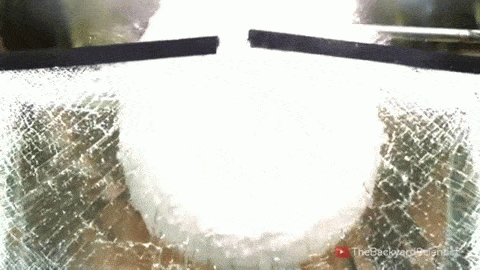
Leidenfrost drops – droplets deposited onto a surface much hotter than their boiling point – are known for their mobility. With the right surface, they can be propelled, trapped, and even guided through a maze, typically by directing the vapor layer that cushions them. But new work shows that these drops have internal dynamics that also contribute to their propulsion.
By adding tracer particles to each droplet, researchers can visualize flows inside the droplet. Large drops tend to have a flatter shape and contain two or more rotating vortices. Such drops won’t propel themselves without another force in play. But smaller droplets are more spherical and contain only a single rotating flow. Once these drops detach, they roll away! Despite the similarity to wheels, these liquid drops aren’t moving the same way. Remember that the drop is not actually in contact with the surface. To see what sets the drop’s direction, researchers examined the shape of the bottom of the drop. They found that it sits at a slant on its vapor cushion. That pushes evaporating gases out one side, propelling the drop the other way. (Image and video credit: A. Bouillant et al., source)