Building sandcastles is more than a pastime for the bumblebee-mimic digger bee. This species of bee collects water into an abdominal pouch, then uses it to wet sand to help her sculpt her nest. She’ll use the material she digs out to create a protective turret over the nest’s entrance, and once her eggs are laid and stocked with food, she’ll deconstruct the turret to rebury the nest and keep her brood safely hidden. (Image and video credit: Deep Look)
Tag: porous media

Chaotic Mixing in Porous Media
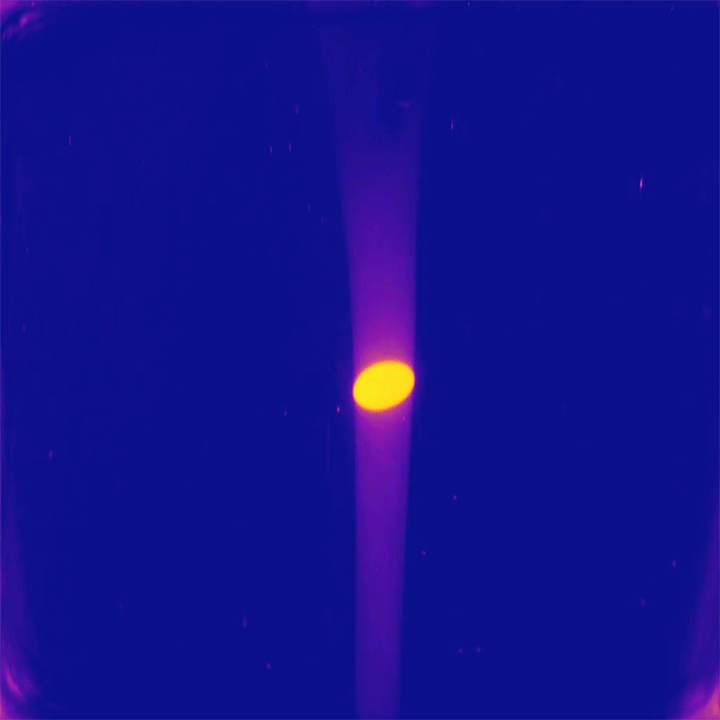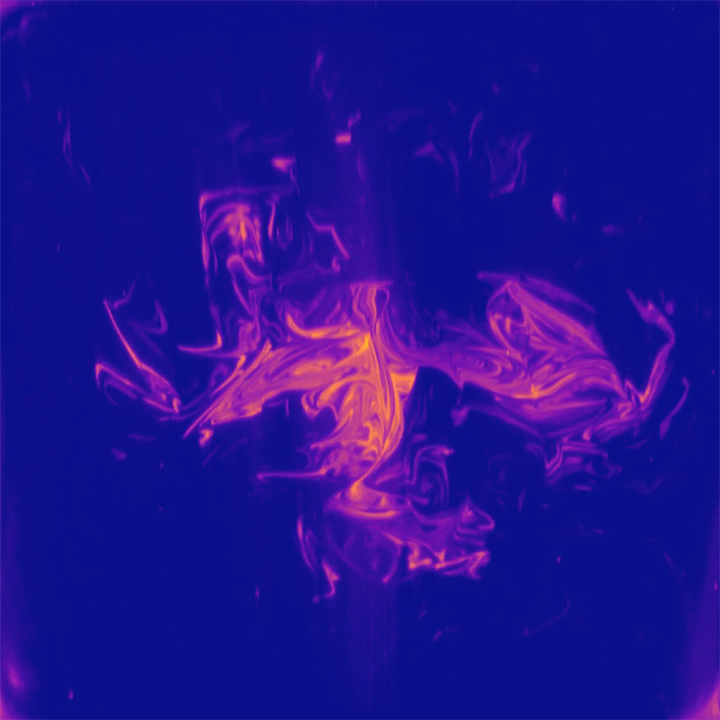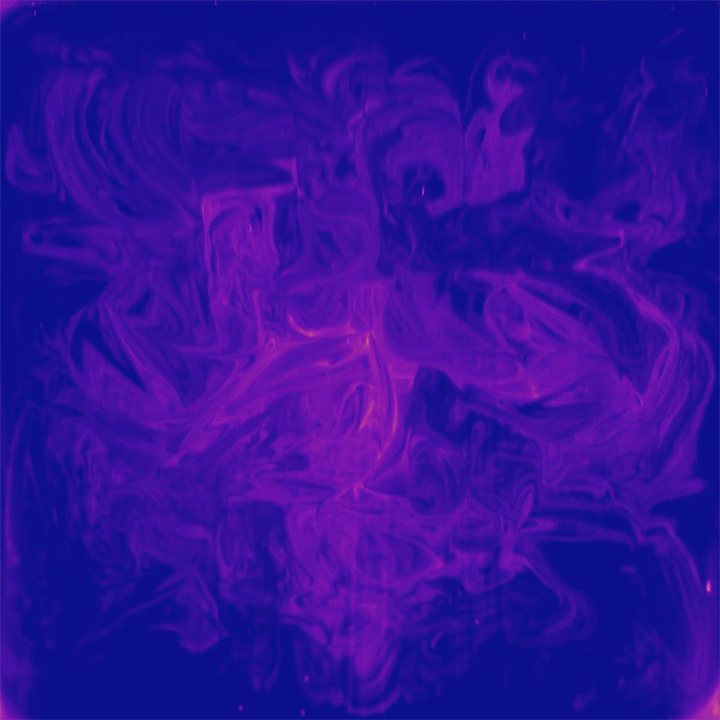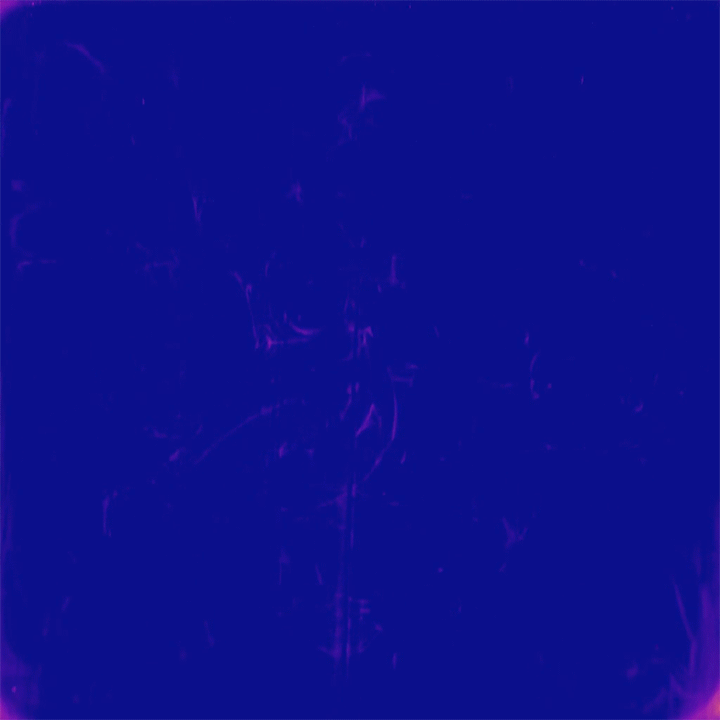
One of the peculiar characteristics of viscous, laminar flows is that they are reversible. Squirt dye into glycerin, stir it one way, then the opposite direction, and the dye returns to its initial position. But this neat trick only works in simple geometries; in a more complex environment, like the pores between packed gravel, flows cannot make their way back to their initial state.
That’s the idea at the heart of this new study of mixing in porous media. Researchers took a bed of packed beads and pushed a slow, steady flow of dye into the bed. Then they steadily withdrew fluid to reverse the flow and observed how the dye they’d injected appeared at the surface of the bed (top image). If the flow were perfectly reversible, we’d expect the dye to return to its injection point. But instead the dye is spread chaotically across the surface, giving researchers a snapshot of the chaotic mixing taking place between beads. (Image and research credit: J. Heyman et al.; via APS Physics)

Inside Drying Wood
Wood must dry before it can be used in most applications, but with its complex internal structure exactly how wood dries out has been unclear. New experiments combining MRI and x-ray imaging reveal a process quite different than expected.
Inside hardwoods like poplar — the species studied here — wood contains both solid structures and pores where water can gather. The pores do not form a fully interconnected network, so capillary action alone is unable to carry water through the pores and out to a surface where it can evaporate.
Instead, researchers found that water evaporating at the surface came from so-called “bound water” in the wood’s solid structures. As the bound water evaporated, it caused water in the wood pores to diffuse into the solid walls, becoming bound and continuing to feed the evaporation. (Image and research credit: H. Penvern et al.; via APS Physics)

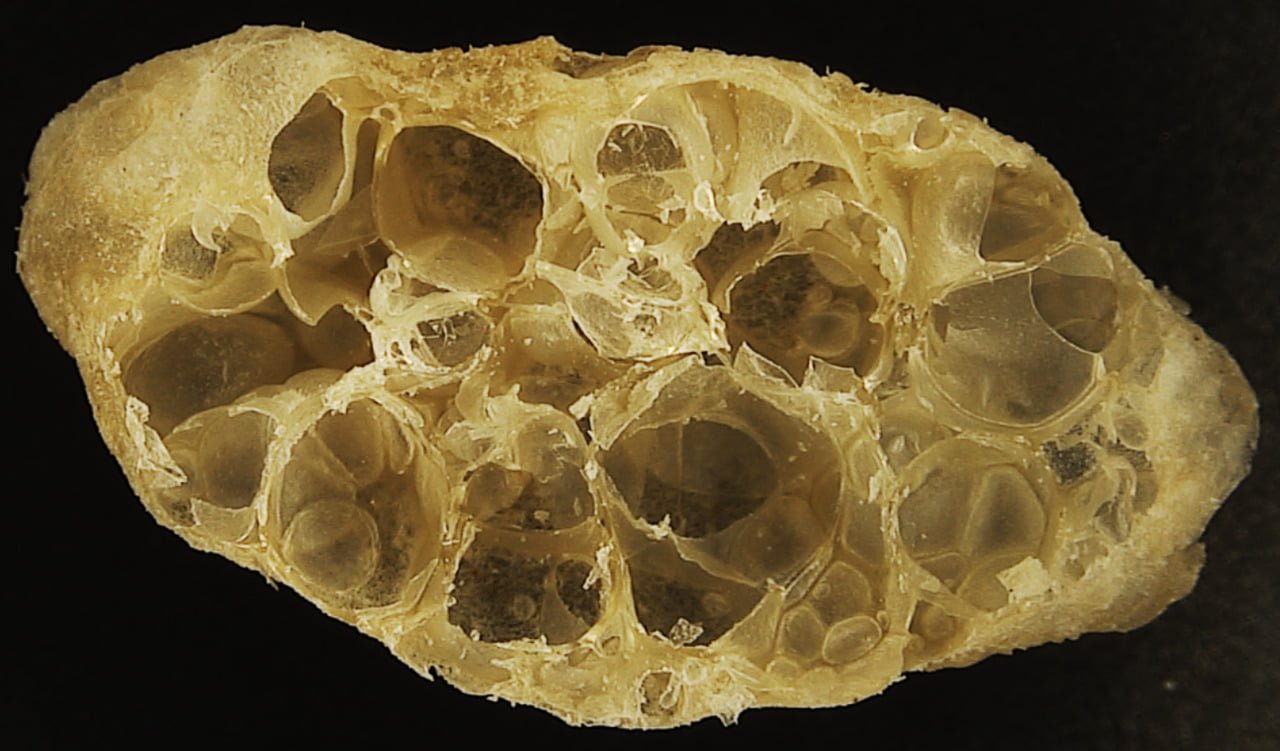
Permeable Pavement
Controlling storm water is a major challenge in urban environments, where many surfaces are impermeable. In a city, rain cannot simply soak into the ground and filter into the water table. One potential solution is permeable pavement, which uses the same ingredients as its common counterpart minus the sand that usually packs into gaps between the gravel. Without the sand, the final pavement allows water to soak through, as seen above. In practice, the water sinks into a porous reservoir beneath the pavement that helps store and regulate the water’s discharge into the soil.
Unfortunately, this solution has its limitations. Permeable pavement is not as strong as the regular variety, so it doesn’t work for highly trafficked areas like roadways. It’s also not well-suited to colder areas, where freezing and thawing may disrupt its operation. But it is another tool in engineers’ toolboxes when it comes to keeping urban environments in harmony with nature’s needs. (Image and video credit: Practical Engineering)

Vanishing Spirits: Aging
The necessary ingredients for scotch whisky’s evaporation patterns are alcohol, surfactants, and polymers; some of those components are absorbed during the spirit’s aging in oak casks. Photographer Ernie Button explored how long it takes for whisky to absorb enough of these chemicals by photographing the stains left by samples aged between 1 and 5 weeks in an oak cask. He found that it takes about 5 weeks for the scotch patterns to begin emerging.
The aging process for scotch and other cask-aged spirits depends on the fluid’s flow through the porous grain of the oak. Evaporation plays a significant role in the process, so the aging process differs based on environmental conditions. For example, distillers in the dry, high-altitude climate of Colorado must use climate-controlled storage, whereas Scottish distillers use a more humid natural climate to their advantage.
Another major factor in the aging process is the charred oak cask itself. Some whiskys, like American bourbon, always use a brand new barrel, whereas scotch is often aged in a previously-used cask. With older casks, absorption of molecules from the wood takes longer, which is why scotch is typically aged for much longer than some other types of whisky. (Image, research, and submission credit: E. Button; see also)

In Search of a Better Espresso
Of specialty coffee drinks, espresso has the most cup-to-cup variation in quality. For those who are not coffee aficionados — such as yours truly — espresso is made by forcing hot water through a packed bed of coffee grains. Many factors can affect the final output, including the amount of dry coffee used, the fineness of the grind, water temperature and pressure, and how tightly packed the granular bed is.
Conventional wisdom suggests that a fine grind is best since it increases the exposed surface area of coffee, but researchers found this is not, in fact, ideal. At very fine grinds, the bed of coffee becomes so tightly packed that water cannot pass through some sections, meaning that the coffee there is completely wasted since nothing is extracted.
Instead, a slightly coarser grind provided better and more consistent extraction because water passed through the entire bed of grains. The researchers point out that this not only produces a good, consistent cup of espresso, but it does so with less waste, something that is becoming more and more important as the climate crisis affects coffee growers. (Image credit: K. Butz; research credit: M. Cameron et al.; via Cosmos; submitted by Kam-Yung Soh)

Ricequakes
Rockfill dams, sinkholes, ice shelves, and other geological features often consist of brittle, porous materials that are partially submerged. Over time, pressure and chemical reactions with the fluid around them can cause these structures to collapse, but it can take many, many years.
To study the physics behind this, researchers have turned to a new model: puffed rice cereal. Like their counterparts in nature, puffed rice grains contain micropores that slowly soften and get crushed after being wetted. Researchers filled their test container with puffed rice and put it under pressure to give the whole stack a constant stress. Then they injected milk in the bottom section of the container. After an immediate collapse in the wet material (lower left), the remaining grains collapsed slowly in a series of “ricequakes”.
As the micropores compacted, the cereal let out audible cracks that corresponded with the motion of a crushing wavefront (lower right). The time between ricequakes increased linearly and depended on pore size. The relationship was so consistent, researchers found, that they could predict how long the puffed rice stack had been wet simply by listening to the time between crackles! Experiments like these offer scientists an exciting chance to understand geological physics that would otherwise take up to millions of years to observe. (Image and research credit: I. Einav and F. Guillard; via Physics World; submitted by Kam-Yung Soh)

Bubble Trains in a Microchannel
Trains of bubbles flowing through a microchannel get distorted by periodic expansions and constrictions. In these images, flow is from left to right, and the narrow point of the channel is about 250 microns across. In narrow regions, the front of the bubble tends to move faster, while in wider areas, the back of the bubble speeds up. This causes the distinctive shape changes we see. Microfluidic channels with these exaggerated shifts in geometry allow researchers to study the physics behind liquids and gases seeping through the interstitial gaps of a porous media, like when water and gases move through rock and soil. (Image and research credit: M. Sauzade and T. Cubaud)

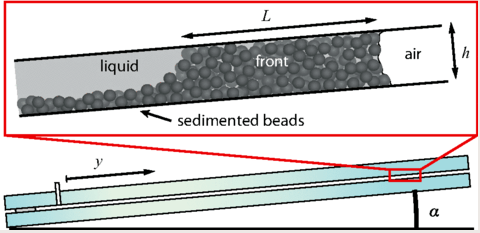
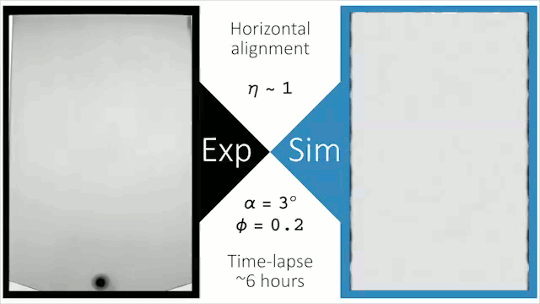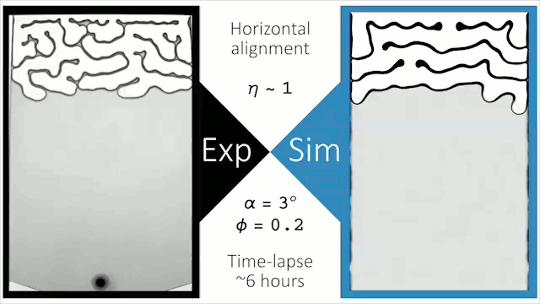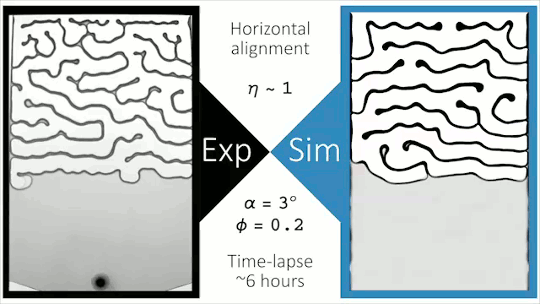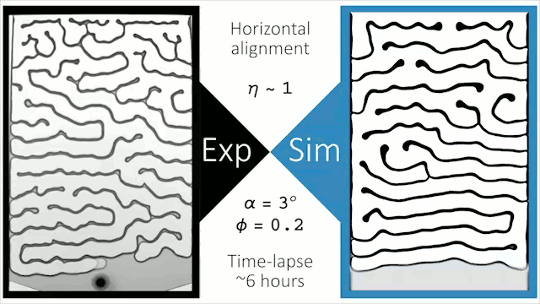
Flowing Through Tight Spaces
Fluid flow through porous media inside confined spaces can be tough to predict but is key to many geological and industrial processes. Here researchers examine a mixture of glass beads and water-glycerol trapped between two slightly tilted plates. As liquid is drained from the bottom of the cell, air intrudes. Loose grains pile up along the meniscus and get slowly bulldozed as the air continues forcing its way in. The result is a labyrinthine maze formed by air fingers of a characteristic width. The final pattern depends on a competition between hydrostatic pressure and the frictional forces between grains. Despite the visual similarity to phenomena like the Saffman-Taylor instability, the authors found that viscosity does not play a major role. For more, check out the video abstract here. (Image and research credit: J. Erikson et al., source)

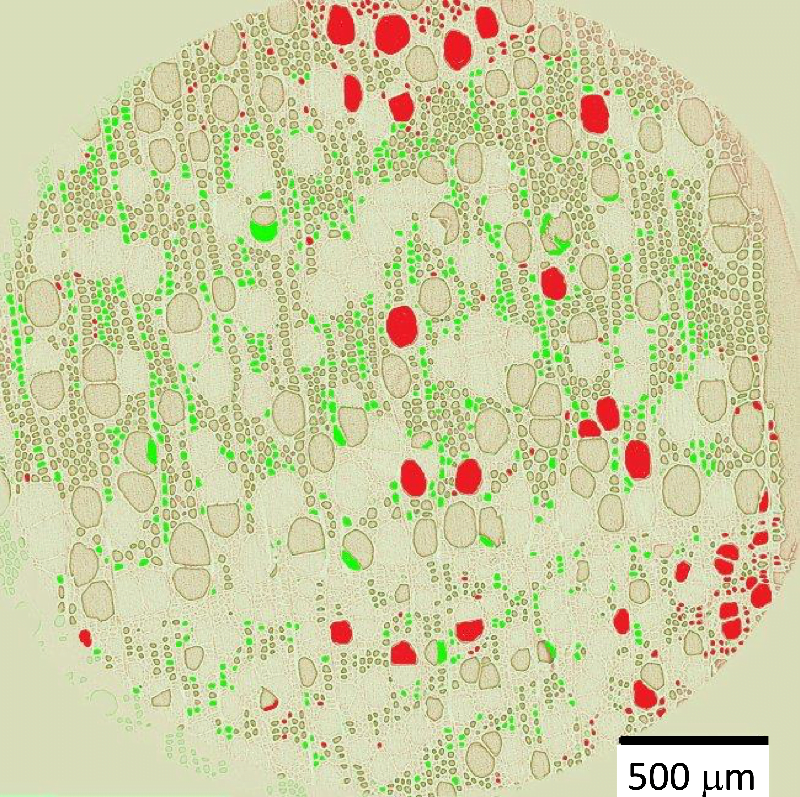
Peering Between Particles
Turbulence is not the only way to mix fluids. Even a steady, laminar flow can be an effective mixer if geometry lends a hand. Above, two dyes, fluorescein (green) and rhodamine (red), are injected into a porous flow through packed spheres. The flow runs from bottom to top in both images. Seeing the flow in such a crowded geometry is challenging. Here researchers used spheres with an index of refraction that matches water – that helps them avoid refraction that would prevent them from looking through spheres to the flow on the other side. They also lit a narrow plane of the flow using a laser sheet to isolate it. Together, this allowed the researchers to track the mixing of the two initially separate streaks of dye as they randomly mix in the spaces between spheres. (Image and research credit: M. Kree and E. Villermaux)