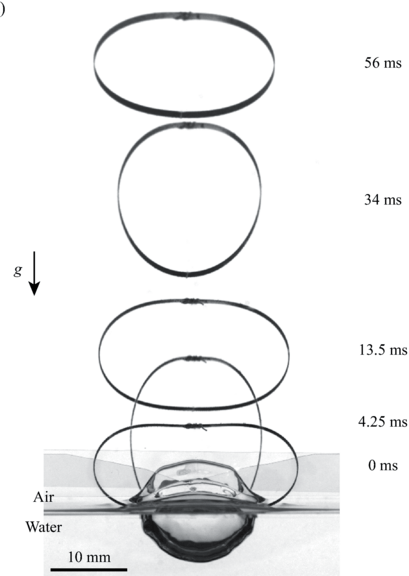
Small creatures like springtails and spiders can jump off the air-water interface using surface tension. But larger creatures can water-jump, too, using drag. Here, researchers study drag-based water jumping with a simple elastic hoop. Initially, two sides of the hoop are pulled closer by a string, deforming the hoop. Then, with the hoop sitting upright on the air-water interface, a laser burns the string, releasing the energy stored in the hoop. The hoop’s bottom pushes into the water, generating drag. That resistance provides a reaction force strong enough to launch the hoop.
Compared to the hoop’s jumps off land, it’s slower to take-off from water, and it’s less efficient at jumping. Lighter hoops, however, jump better off water than heavier ones — a wrinkle that isn’t seen in ground jumpers. That suggests that weight reduction is more important for aquatic jumpers than for their terrestrial counterparts. (Image and research credit: H. Jeong et al.)