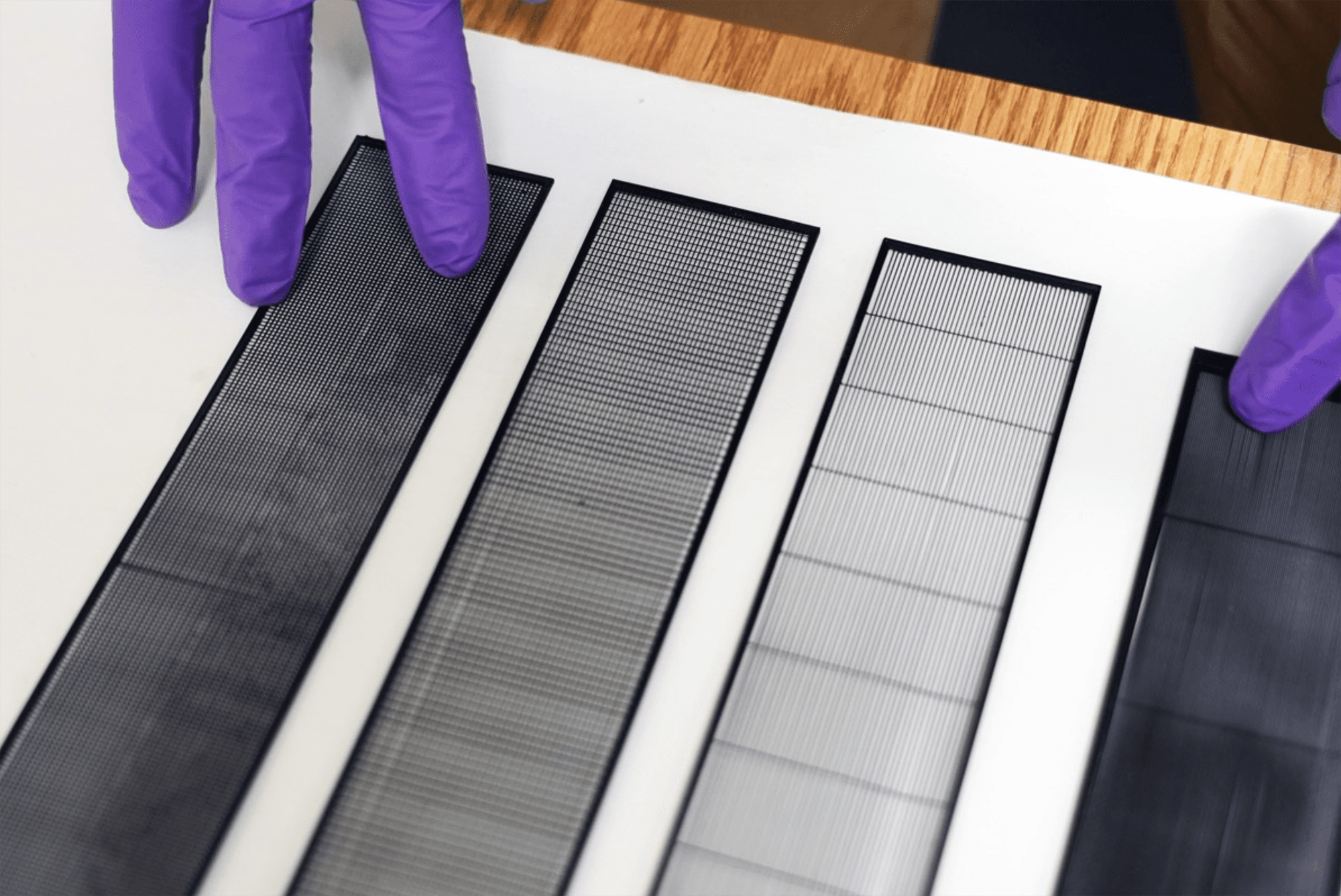
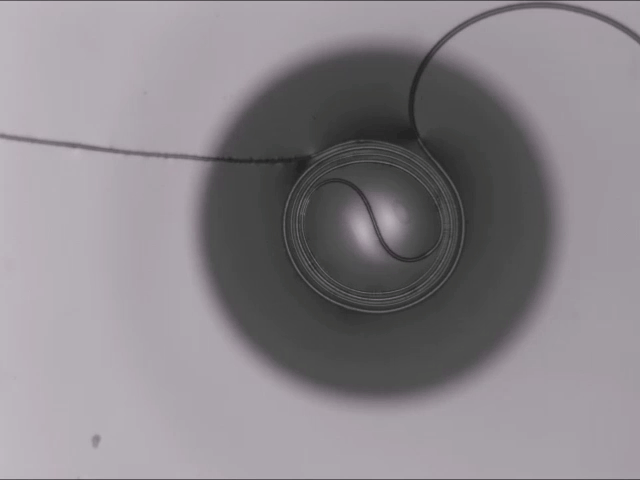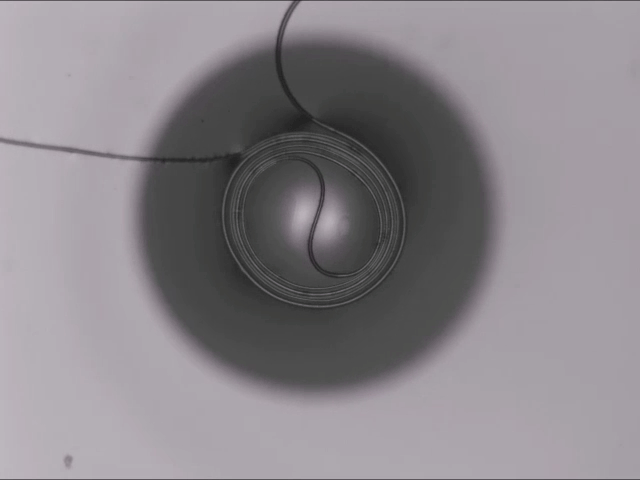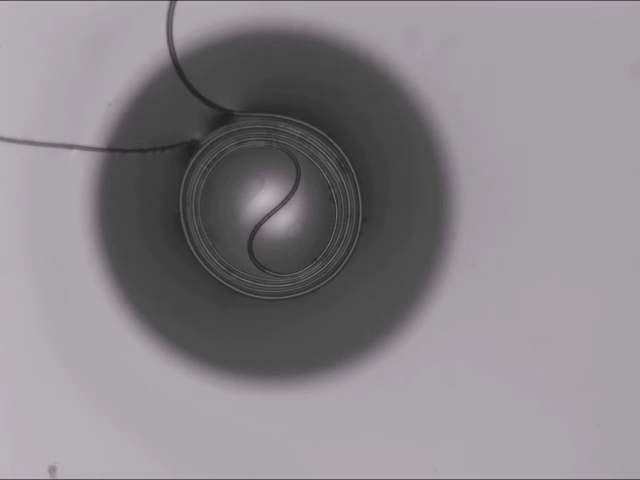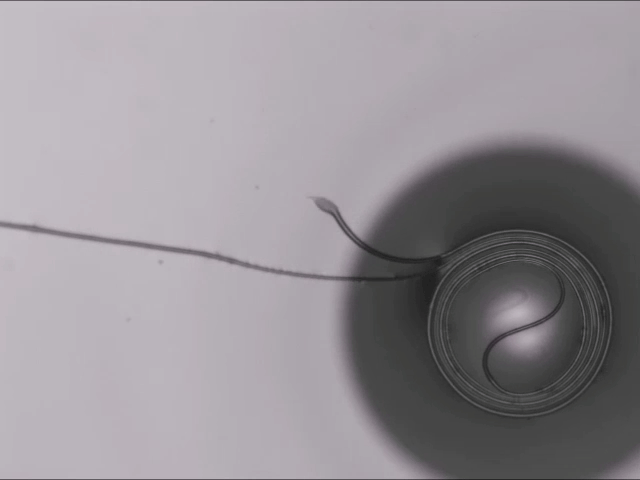
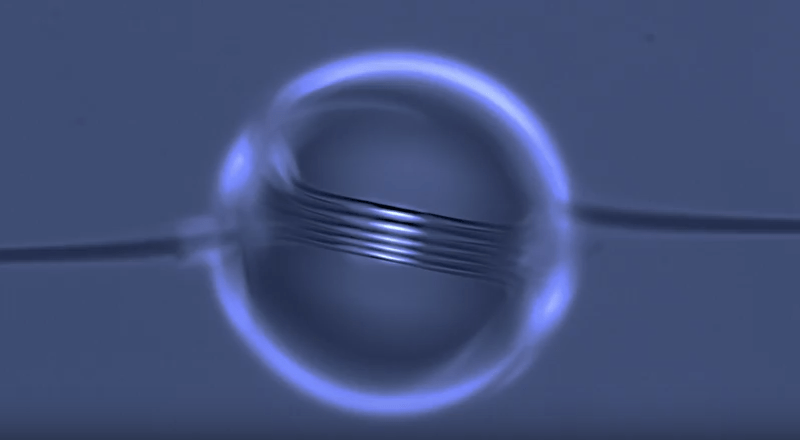
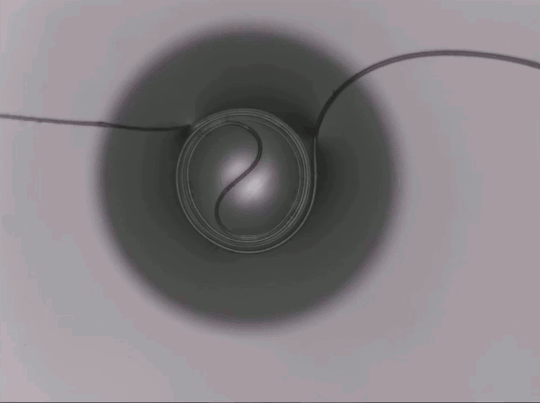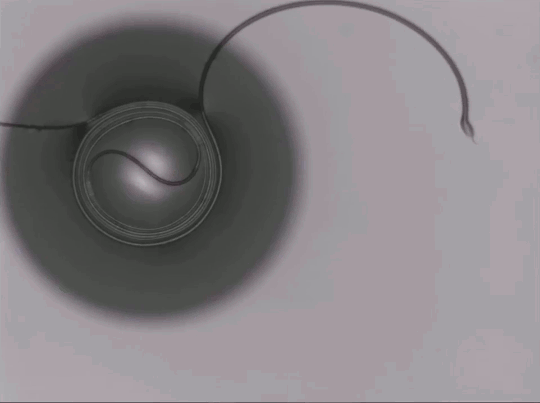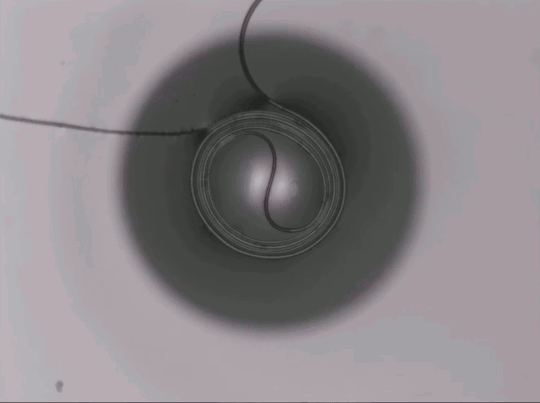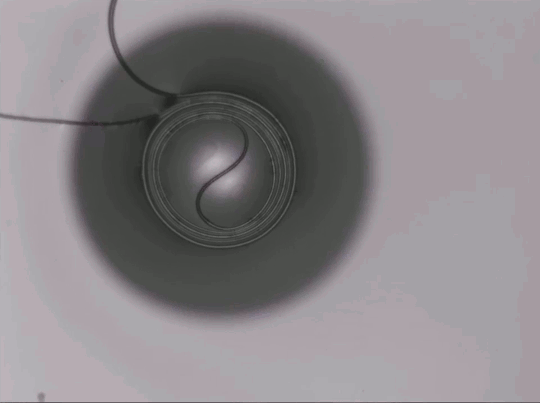
On arid coastlines, fog rolling in can serve as an important water source. Today’s fog collectors often use tight mesh nets. The narrow holes help catch tiny water particles, but they also clog easily. A few years ago, researchers suggested an alternative design — a fog harp inspired by coastal redwoods — that used closely spaced vertical wires to capture water vapor. At small scales, this technique worked well, but once scaled up to a meter-long fog harp, the strings would stick together once wet — much the way wet hairs cling to one another.
The group has iterated on their design with a new hybrid that maintains the fog harp’s close vertical spacing but adds occasional cross-wires to stabilize. Laboratory tests are promising, with the new hybrid fog harp collecting water with 2 – 8 times the efficiency of either a conventional mesh or their original fog harp. The team notes that even higher efficiencies are possible with electrification. (Image credit: A. Parrish; research credit: J. Kaindu et al.; via Ars Technica)