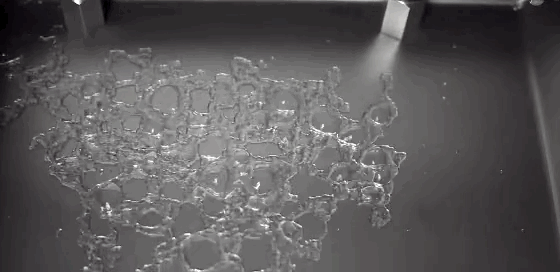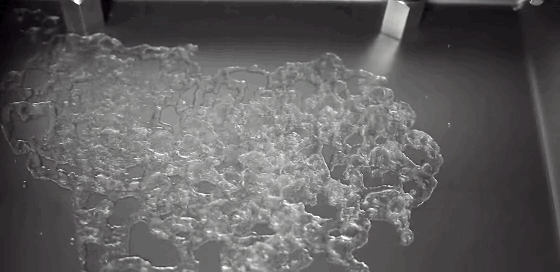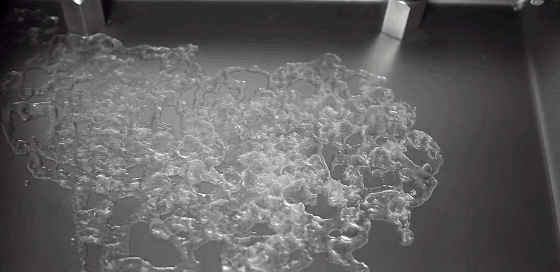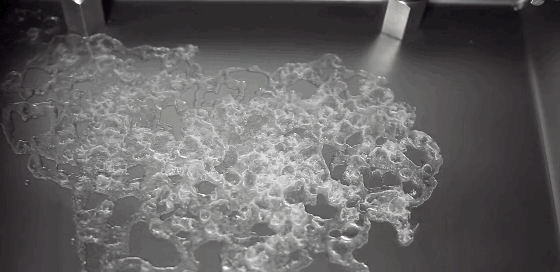Last November, a windstorm, known as Storm Ciarán in the U.K., blew through Europe with wind speeds as high as 130 kilometers per hour. All that wind came with a significant drop in atmospheric pressure. Researchers found that the pressure drop was large enough to lower the boiling point of water more than full 2 degrees Celsius. That difference probably wouldn’t register for anyone waiting for their kettle to boil, but it could decidedly affect the final cup of tea. Tea flavor is quite sensitive to the temperature of the boiling water used to brew it, as it affects how well the tannins get extracted. According to the researchers, Ciarán’s conditions potentially ruined millions of cups of breakfast tea in the greater London area. (Image credit: E. Akyurt; research credit: G. Harrison et al.; via Gizmodo)
Tag: boiling point

Does Liquid in a Vacuum Boil or Freeze?
What happens to a liquid in a cold vacuum? Does it boil or freeze? These animations of liquid nitrogen (LN2) in a vacuum chamber demonstrate the answer: first one, then the other! The top image shows an overview of the process. At standard conditions, liquid nitrogen has a boiling point of 77 Kelvin, about 200 degrees C below room temperature; as a result, LN2 boils at room temperature. As pressure is lowered in the vacuum chamber, LN2’s boiling point also decreases. In response, the boiling becomes more vigorous, as seen in the second row of images. This increased boiling hastens the evaporation of the nitrogen, causing the temperature of the remaining LN2 to drop, the same way sweat evaporating cools our bodies. When the temperature drops low enough, the nitrogen freezes, as seen in the third row of images. This freezing happens so quickly that the nitrogen molecules do not form a crystalline lattice. Instead they are an amorphous solid, like glass. As the residual heat of the metal surface warms the solid nitrogen, the molecules realign into a crystalline lattice, causing the snow-like flakes and transition seen in the last image. Water can also form an amorphous ice if frozen quickly enough. In fact, scientists suspect this to be the most common form of water ice in the interstellar medium. (GIF credit: scientificvisuals; original source: Chef Steps, video; h/t to freshphotons)

The Leidenfrost Effect
The Leidenfrost effect occurs when a liquid comes in contact with a mass significantly hotter than the liquid’s boiling point. Upon contact, a thin layer of the liquid will be vaporized, forming a lubricating gas layer that temporarily insulates the hot mass from the cold liquid. This effect is responsible for water skittering across a hot plate as well as protecting the hands of many a professor from a dunk in liquid nitrogen at the front of a classroom.
reblogged from fyeahchemistry:
(Thanks for the submission, singbird-sing!)