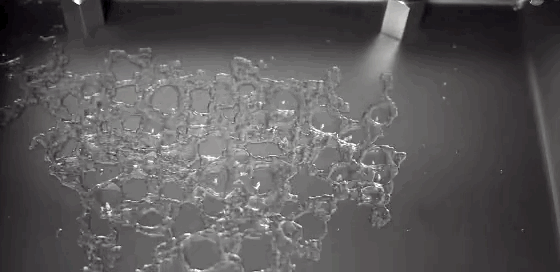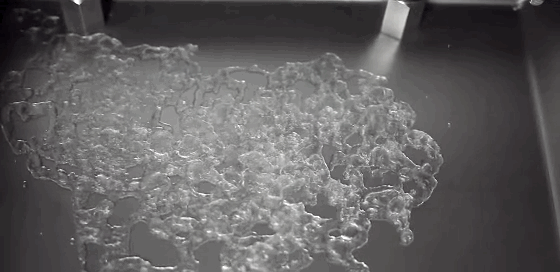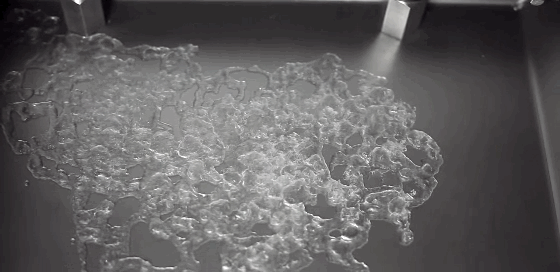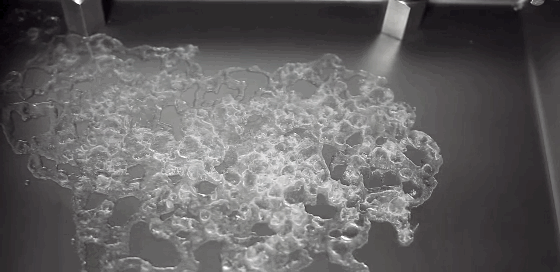As children, we’re taught that there are three distinct phases of matter–solid, liquid, and gas–but the reality is somewhat more complicated. In the right–often exotic–conditions, there are far more phases matter takes on. In a recent study, researchers described a metal that sits somewhere between a liquid and a solid.
In a liquid, atoms are free to move. During solidification, atoms lose this freedom, and their frozen positions relative to one another determine the solid’s properties. Atoms frozen into orderly patterns form crystals, whereas those frozen haphazardly become amorphous solids. In their experiment, researchers instead observed atoms in liquid metal nanoparticles that remained stationary throughout the transition from liquid to solid. The number and position of stationary atoms affected whether the final solid crystallized or not.
By tracking these stationary atoms and their influence, the team hopes to better control the material properties of the final solidified metal. (Image credit: U. of Nottingham; research credit: C. Leist et al.; via Gizmodo)