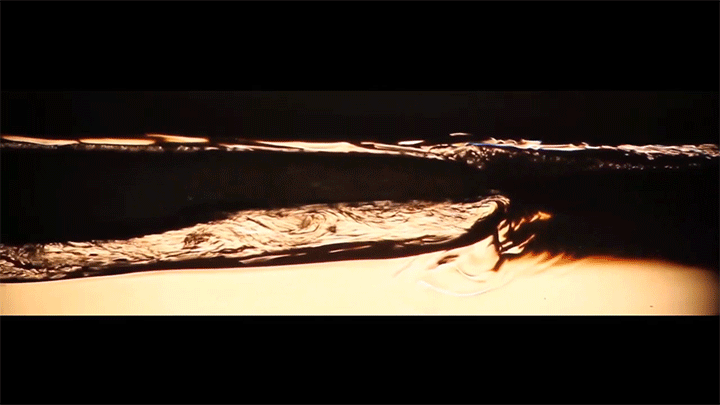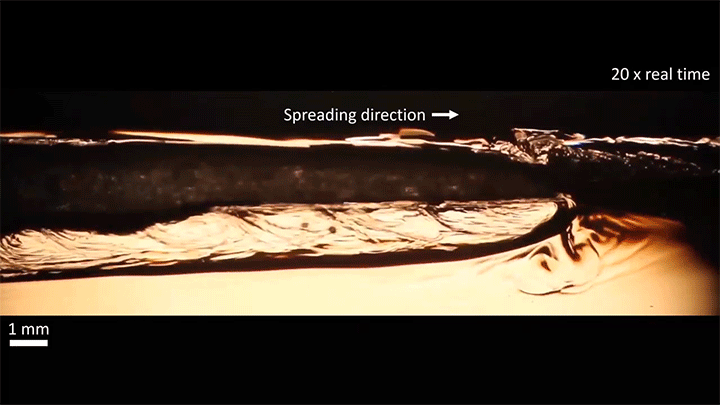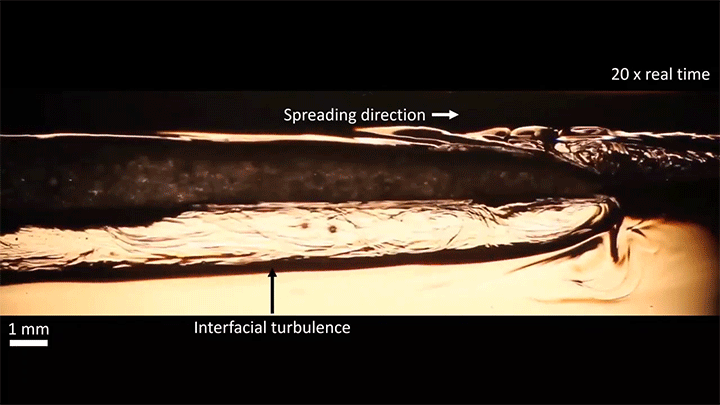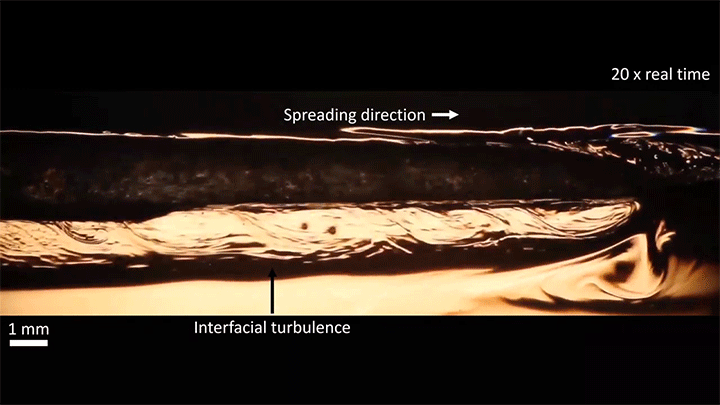
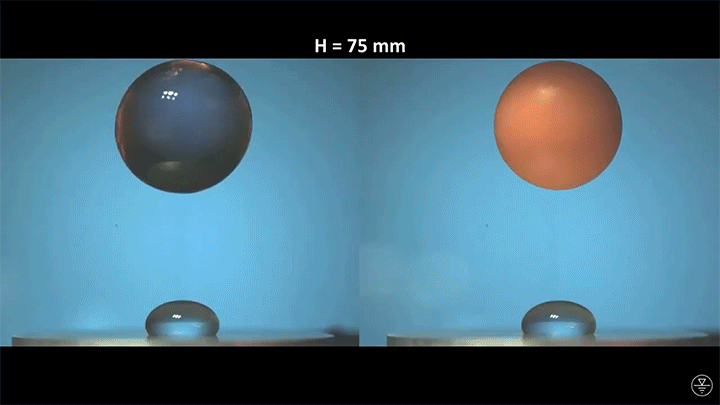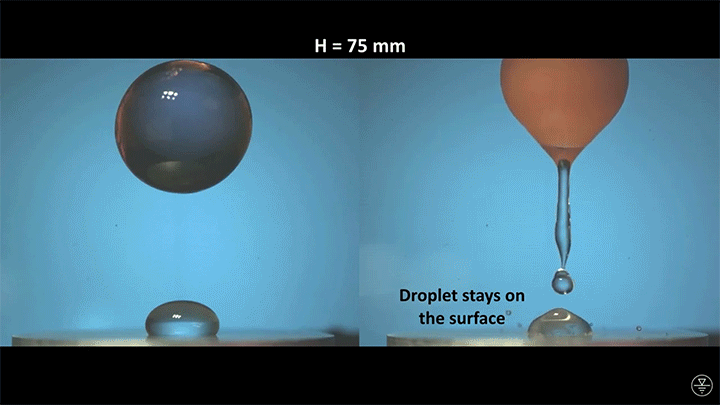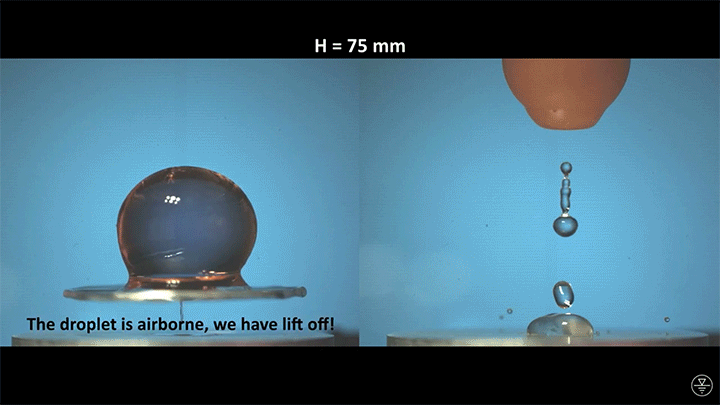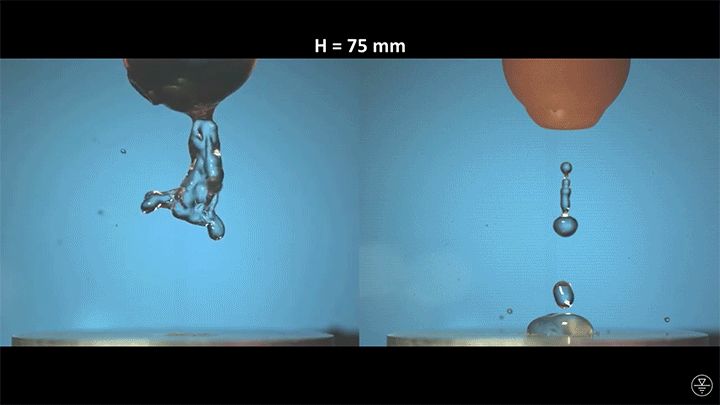
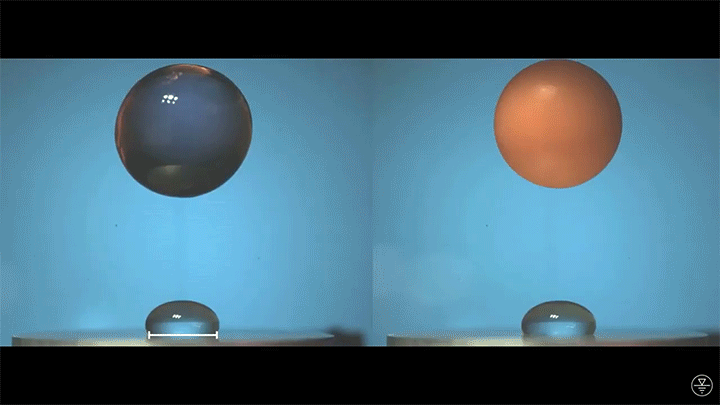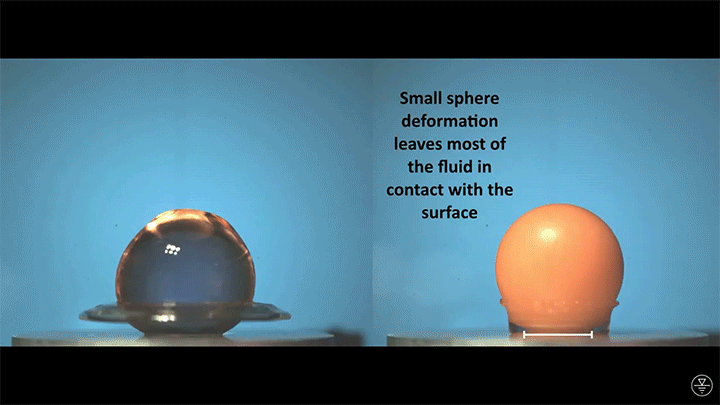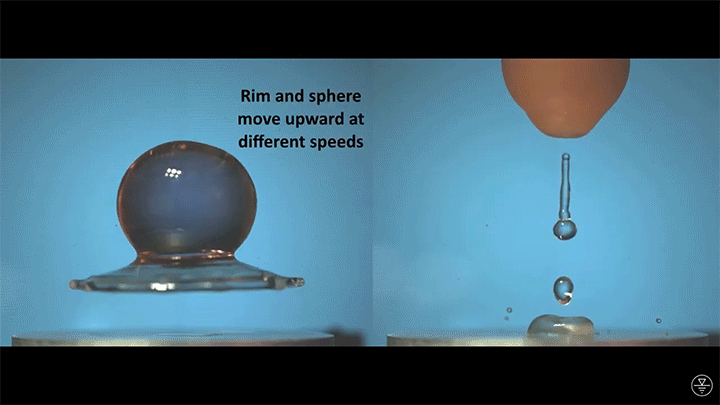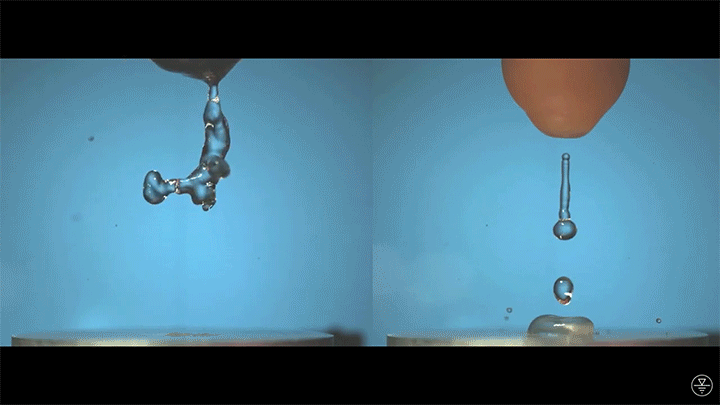
When placed on a very hot, patterned surface, droplets will self-propel on a layer of their own vapor. Here, researchers use this to drive droplets to coalesce so that they can observe how well they mix. After their head-on collision, the merged droplets have two major forces fighting in them: surface tension, which tries to minimize the overall surface area; and gravity, which tries to flatten the large droplet. Together, these forces drive the large oscillations we see in the merged drop, and those oscillations help mix the liquid from the two original drops together. (Image, video, and research credit: Y. Chiu and C. Sun)