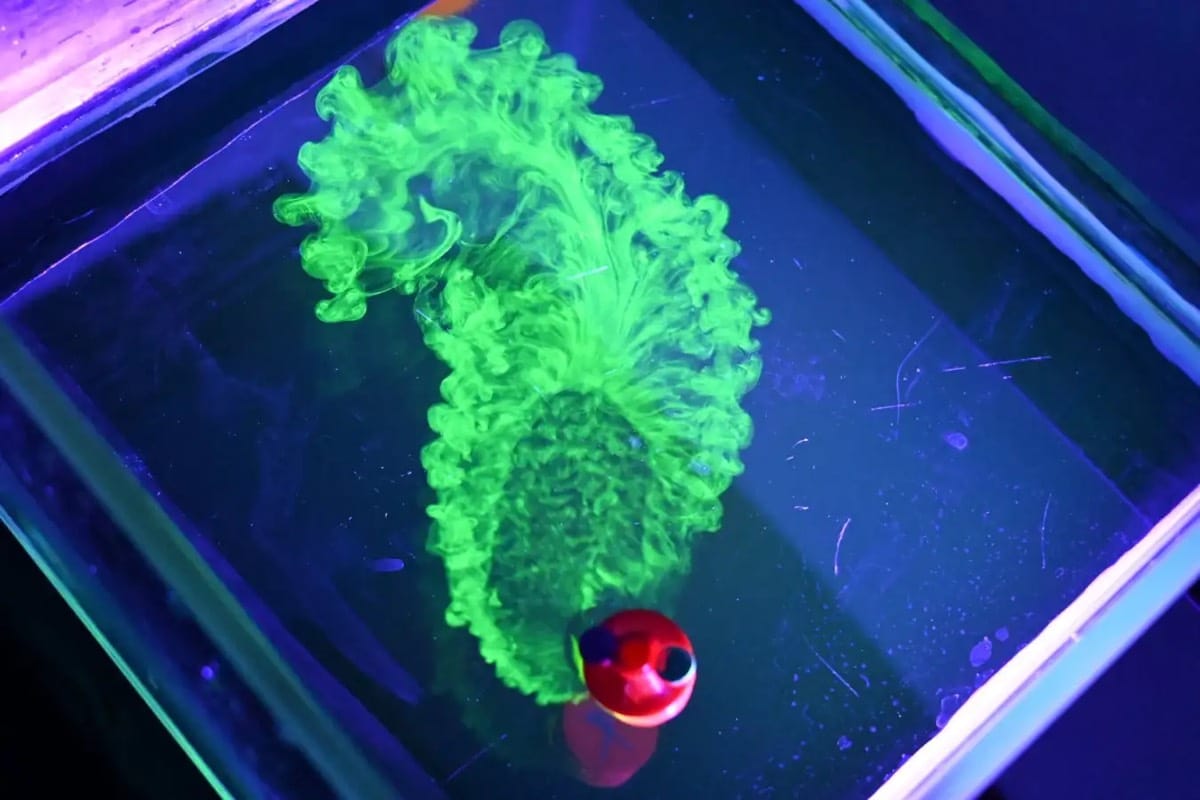
Droplets of a gallium alloy are liquid at room temperature. When spiked with aluminum grains and immersed in a solution of NaOH, the droplets change shape and move in a random fashion. This video delves into the phenomenon, describing how a chemical reaction with the aluminum grains changes the local surface tension and creates Marangoni flows that make the droplets move. To get the droplet motion, you need to have the aluminum concentration just right. With too little, there’s not enough Marangoni flow. With too much, the hydrogen gas produced in the chemical reaction disrupts the droplet motion. (Video and image credit: N. Kim)
Tag: marangoni effect

Hot Droplets Bounce
In the Leidenfrost effect, room-temperature droplets bounce and skitter off a surface much hotter than the drop’s boiling point. With those droplets, a layer of vapor cushions them and insulates them from the hot surface. In today’s study, researchers instead used hot or burning drops (above) and observed how they impact a room-temperature surface. While room-temperature droplets hit and stuck (below), hot and burning droplets bounced (above).
In this case, the cushioning air layer doesn’t come from vaporization. Instead, the bottom of the falling drop cools faster than the rest of it, increasing the local surface tension. That increase in surface tension creates a Marangoni flow that pulls fluid down along the edges of the drop. That flow drags nearby air with it, creating the cushioning layer that lets the drop bounce. In this case, the authors called the phenomenon “self-lubricating bouncing.” (Image and research credit: Y. Liu et al.; via Ars Technica)


“Trinity”
Inspired by the film Oppenheimer, artist Thomas Blanchard created “Trinity,” a short film imagining a nuclear explosion with macro-scale fluid motion. There’s clever video editing and compositing in this video, but no CGI. Instead, Blanchard filmed fire, sparklers, alcohol inks, pigments and more up close and in stunning detail. As always, his work is a reminder of the amazing possibilities of analog-based art. (Video and image credit: T. Blanchard)

Within a Drop
In this macro video, various chemical reactions swirl inside a single dangling droplet. Despite its tiny size, quite a lot can go on in a drop like this. Both the injection of chemicals and the chemical reactions themselves can cause the flows we see here. Surface tension variations and capillary waves on the exterior of the drop can play a role, too. Just because a flow is tiny doesn’t mean it’s simple. (Video and image credit: B. Pleyer; via Nikon Small World in Motion)

Chemical reactions swirl within a single, hanging droplet. 
Active Cheerios Self-Propel
The interface where air and water meet is a special world of surface-tension-mediated interactions. Cereal lovers are well-aware of the Cheerios effect, where lightweight O’s tend to attract one another, courtesy of their matching menisci. And those who have played with soap boats know that a gradient in surface tension causes flow. Today’s pre-print study combines these two effects to create self-propelling particle assemblies.
The team 3D-printed particles that are a couple centimeters across and resemble a cone stuck atop a hockey puck. The lower disk area is hollow, trapping air to make the particle buoyant. The cone serves as a fuel tank, which the researchers filled with ethanol (and, in some cases, some fluorescent dye to visualize the flow). Like soap, ethanol’s lower surface tension disrupts the water’s interface and triggers a flow that pulls the particle toward areas with higher surface tension. But, unlike soap, ethanol evaporates, effectively restoring the interface’s higher surface tension over time.
With multiple self-propelling particles on the interface, the researchers observed a rich series of interactions. Without their fuel, the Cheerios effect attracted particles to each other. But with ethanol slowly leaking out their sides, the particles repelled each other. As the ethanol ran out and evaporated, the particles would again attract. By tweaking the number and position of fuel outlets on a particle, the researchers found they could tune the particles’ attractions and motility. In addition to helping robots move and organize, their findings also make for a fun educational project. There’s a lot of room for students to play with different 3D-printed designs and fuel concentrations to make their own self-propelled particles. (Research and image credit: J. Wilt et al.; via Ars Technica)

“Alive Painting”
Artist Akiko Nakayama’s intuitive grasp of fluid dynamics is so good that she manipulates liquids live to musical accompaniment. Her dendritic paintings — made from a combination of acrylic paint and isopropyl alcohol — inspired scientific research papers. There’s no substitute, I’m sure, for seeing her art live, but you can get a taste of her performances in the video below. Then you can head over to Physics World for more on the artist, her inspirations, and her scientific collaborations. (Image credits: H. Akagi and A. Nakayama; video credit: Eternal Art Space; via Physics World)

“Chemical Somnia”
Under a macro lens, even a petri dish worth of fluids comes vividly to life. Here, artist Scott Portingale explores crystallization, Marangoni effects, and other phenomena alongside a haunting soundtrack from musician Gorkem Sen. Enjoy! (Image and video credit: S. Portingale et al.)

Marangoni Blossoms
When surface tension varies along an interface, fluids move from regions of low surface tension to higher surface tension, a behavior known as the Marangoni effect. Here, a drop of (dyed) water is placed on glycerol. The two fluids are miscible, but water has much a lower viscosity and density yet a higher surface tension. The drop’s interface quickly becomes unstable; viscous fingers form along the edge as the less viscous water pushes into the more viscous glycerol. Eventually, the surface-tension-driven Marangoni flow breaks those fingers off into lip-like daughter drops. The researchers also show how the interplay between viscosity and surface tension affects the size of fingers that form by varying the water/glycerol concentration. (Image and video credit: A. Hooshanginejad et al.)

“Color Show”
Brightly colored paints and inks mix and flow in artist Roman De Giuli’s “Color Show.” De Giuli typically creates this fluid art in thin layers atop paper. He’s a master of the form, manipulating surface tension gradients to create streaming flows, dendritic patterns, and feathery wisps. If this kind of art is your jam, he offers an app full of live wallpapers* for Android phones. See more of his work on his website and on Instagram. (Video and image credit: R. De Giuli)
*Not sponsored, I just like his art!
Dendritic Painting Physics
In the art of Akiko Nakayama, colors branch and split in a tree-like pattern. In studying the process, researchers found the physics intersected art, soft matter mechanics, and statistical physics. In dendritic painting, the process starts with an underlying layer of acrylic paint, diluted with water. Atop this wet layer, you place a drop of acrylic ink mixed with isopropyl alcohol.
The combination of both layers is key. The alcohol-acrylic drop on a Newtonian substrate will show spreading, driven by Marangoni forces, but no branching. It’s the slightly shear-thinning nature of the diluted acrylic paint substrate that allows dendrites to form. As the overlying drop expands, it shears the underlayer, changing its viscosity and allowing the branches to form. You can see video of the process here. (Image credit: A. Nakayama; research credit: S. Chan and E. Fried; via Physics World)