Superhydrophobic surfaces repel water. Both naturally occurring and manmade materials with this property share a common feature: micro- or nanoscale structures on their surface. Lotus and lily leaves are coated with tiny hairs, and synthetic coatings or micro-manufactured surfaces like the one in the video above can be made in the lab. This nanoscale roughness traps air between the surface and the water, preventing adhesion to the surface and enabling the water-repelling behavior we observe at the human scale. Although effective, these nanoscale structures are also extremely delicate, which makes widespread application of superhydrophobic coatings and textures difficult. (Video credit: G. Azimi et al.)
Tag: superhydrophobic

The March of Drops
I love science with a sense of humor. This video features a series of clips showing the behavior of droplets on what appears to be a superhydrophobic surface. In particular, there are some excellent examples of drops bouncing on an incline and droplets rebounding after impact. For droplets with enough momentum, impact flattens them like a pancake, with the rim sometimes forming a halo of droplets. If the momentum is high enough, these droplets can escape as satellite drops, but other times the rebound of the drop off the superhydrophobic surface is forceful enough to overcome the instability and draw the entire drop back off the surface. (Video credit: C. Antonini et al.)

Rebounding
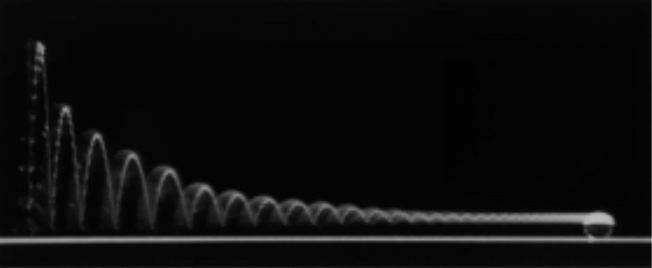
A water droplet can rebound completely without spreading from a superhydrophobic surface. The photo above is a long exposure image showing the trajectory of such a droplet as it bounces. In the initial bounces, the droplet leaves the surface fully, following a parabolic path with each rebound. The droplet’s kinetic energy is sapped with each rebound by surface deformation and vibration, making each bounce smaller than the last. Viscosity damps the drop’s vibrations, and the droplet eventually comes to rest after twenty or so rebounds. (Image credit: D. Richard and D. Quere)

Water and Aerogel
Aerogel is an extremely light porous material formed when the liquid inside a gel is replaced with gas. When combined with water, aerogel powders can have some wild superhydrophobic effects. Here water condensed on a liquid nitrogen cooler has dripped onto a floor scattered with aerogel powder from the nitrogen’s shipping container. The result is that the water gets partially coated in aerogel powder and takes on some neat properties. Its contact angle with the surface increases – in other words, it beads up – which is typical of superhydrophobicity. When disturbed, the water breaks easily into droplets which do not immediately recombine upon contact. With sufficient distortion, they can rejoin. You can see some other neat examples of aerogel-coated water behaviors in this second video as well. (Video credit: ophilcial; submitted by Jason I.)

Fluids Round-up – 11 January 2014
It’s a big fluids round-up today, so let’s get right to it.
- Over at txchnologist, there’s a great article on controlling combustion instabilities in rocket engines with sound.
- Quanta Magazine asks if knot theory can help unravel turbulence. (submitted by iamaponyrocket)
- SciAm takes a look at how FIFA finally got their aerodynamics right so that their video game football (soccer) balls fly correctly.
- The Smithsonian considers an important question: can you fry foods in space?
- The Navy unveiled a fantastic new facility for simulating ocean waves (via J. Ouellette)
- At SciAm, there’s a nice explanation of the polar vortex and its effects on recent freezing weather. For additional background, check out this excerpt from a presentation by meteorology professor Jennifer Francis. (via Nicholas Travers)
- Cold weather also brings a host of new viral videos; NatGeo explains some of the science behind instant snow, ice fog, and frozen bubbles. See also: our own explanation of the instant snow phenomenon.
- io9 looks at the physics of knuckleballs.
- Over at Wired, Rhett Allain questions whether dwarves should stand in floating barrels. Also on the subject of The Hobbit, here’s an analysis of fire-breathing in dragons.
- At SciAm, Kyle Hill explains how inertia lets one pour a drink toward the sky.
- SciAm reports on a manufacturing process for superhydrophobic paper.
- I don’t know what banking has to do with a pool of non-Newtonian fluids, but this Malaysian ad sure makes it look fun. (via physicsphysics and jmlinhart)
- Wired has a great write-up on the mantis shrimp, which kills its prey with cavitation.
- io9 tackles explaining one of the most vexing brain teasers in fluid dynamics, the Feynman sprinkler.
- Finally, today’s lead image comes from our friends at Think Elephants, who study elephant intelligence over in Thailand and occasionally capture the animals’ mastery of fluid dynamics. Be sure to check them out and follow them on Twitter and Facebook.
(Photo credit: Think Elephants International/R. Shoer)

Hydrophobia
On a recent trip to G.E., the Slow Mo Guys used their high-speed camera to capture some great footage of dyed water on a superhydrophobic surface. Upon impact, the water streams spread outward, flat except for a crownlike rim around the edges. Then, because air trapped between the liquid and the superhydrophobic solid prevents the liquid from wetting the surface, surface tension pulls the water back together. If this were a droplet rather than a stream, it would rebound off the surface at this point. Instead, the jet breaks up into droplets that scatter and skitter across the surface. There’s footage of smaller droplets bouncing and rebounding, too. Superhydrophobic surfaces aren’t the only way to generate this behavior, though; the same rebounding is found for very hot substrates due to the Leidenfrost effect and very cold substrates due to sublimation. As a bonus, the video includes ferrofluids at high-speed, too. (Video credit: The Slow Mo Guys/G.E.)

Fluids Round-up – 5 October 2013
This is the last week that my IndieGoGo project is open for donations. All money above and beyond what is needed for the conference will go toward FYFD-produced videos. Also, donors can get some awesome FYFD stickers.
As a reminder, those looking for more fluids–in video, textbook, or other form–can always check out my resources page. And if you know about great links that aren’t on there, let me know so that I can add them. On to the round-up!
- Popular Science has look at what it was like to fly on the Concorde, the only supersonic commercial airliner ever flown.
- For the cyclists and CFD folks out there, Zipp has put out a new video discussing their Firecrest wheels’ aerodynamics.
- io9 explains how superhydrophobic surfaces impart a charge to water droplets and how this can be used to increase efficiency at power plants.
- BuzzFeed UK has 32 fun science GIFs, several of which are fluids-related, and several of which will look familiar to long-time readers. (via Flow Visualization on FB)
- Wired has an intriguing short on Acoustic Archives, a group that focuses on capturing the acoustic qualities of historic locations using custom-designed 3D microphones.
- Congratulations to Richard over at Flow Viz for hitting his 100th post! Here’s to many more.
- Finally, our lead image comes from Martin Klimas. Smithsonian’s blog has a feature on his work in which he transforms songs from artists like Pink Floyd, Daft Punk, and Bach into sonic sculptures using paint on speakers. (via Flow Visualization on FB)
I had a lot of fun earlier this week giving a talk for the Texas A&M Applied Mathematics Undergraduate Seminar series. I didn’t get a chance to record it, but the slides are up here if anyone is interested.(Photo credit: M. Klimas)
Rebounding Jets

The photo sequence in the upper image shows, left to right, a fluid-filled tube falling under gravity, impacting a rigid surface, and rebounding upward. During free-fall, the fluid wets the sides of the tube, creating a hemispherical meniscus. After impact, the surface curvature reverses dramatically to form an intense jet. If, on the other hand, the tube is treated so that it is hydrophobic, the contact angle between the liquid and the tube will be 90 degrees during free-fall, impact, and rebound, as shown in the lower image sequence. The liquid simply falls and rebounds alongside the tube, without any deformation of the air-liquid interface. (Photo credit: A. Antkowiak et al.)

Self-Assembling Ferrofluids
Ferrofluids–colloidal suspensions made up of ferromagnetic nanoparticles and a carrier liquid–are known for their interesting and sometimes bizarre behaviors due to magnetic fields. The video above shows how, when subjected to an increasing magnetic field, a single droplet of a ferrofluid on a superhydrophobic surface will split into several droplets. The process is called static self-assembly, and it results from the ferrofluid seeking a minimum energy state relative to the force supplied by the magnetic field. Change the magnetic field and the droplets shift to the next energy minimum. But what happens when you change the magnetic field continuously and too quickly for the droplets to respond? A whole different set of structures and behaviors are observed (video link). This is dynamic self-assembly, a different ordered state only achieved when the ferrofluid is forceably kept away from the energy minima seen in the first video. For more, see the additional videos and the original paper. (Video credit: J. Timonen et al.; via io9)

“Perpetual Puddle Vortex Experiment”
Anthony Hall’s “Perpetual Puddle Vortex Experiment” is an intriguing display of several physical mechanisms. What looks like a puddle is actually a vortex constantly sucking fluid down a hole in the table. The liquid is re-circulated into the puddle so it never disappears. The table itself is treated to be hydrophobic, causing the distinctive curvature and large contact angle of the puddle’s rim. The oils mixed in float on top, creating patterns of foam that visualize the swirling motions of the fluid as the vortex pulls it in. (Video credit and submission by: A. Hall)