A water droplet immersed in a mixture of anise oil and ethanol displays some pretty complicated dynamics. Its behavior is driven, in part, by the variable miscibility of the three liquids. Water and ethanol are fully miscible, anise oil and ethanol are only partially miscible, and anise oil and water are completely immiscible. These varying levels of miscibility set up a lot of variations in surface tension along and around the droplet, which drives its stretching and eventual jump.
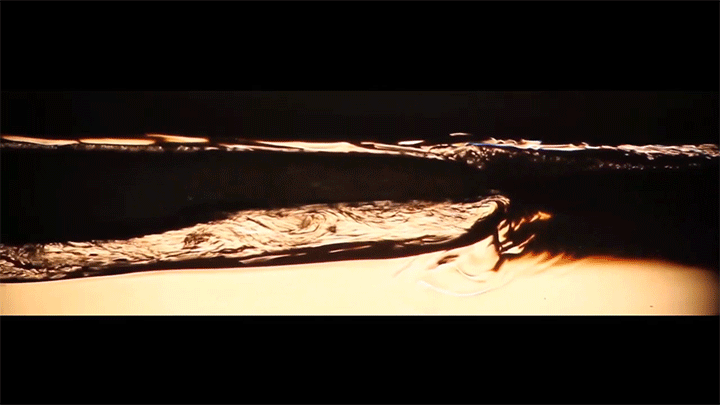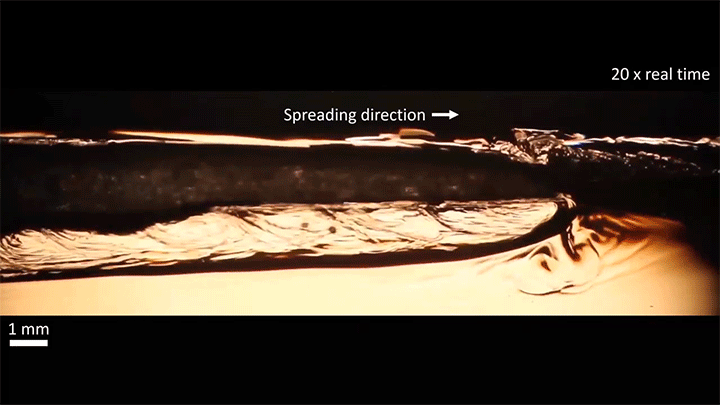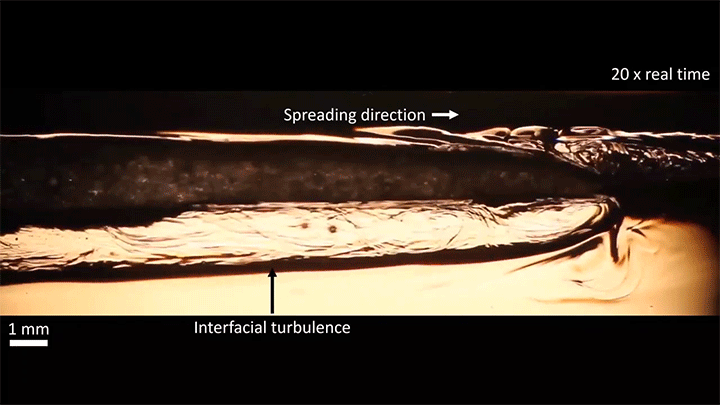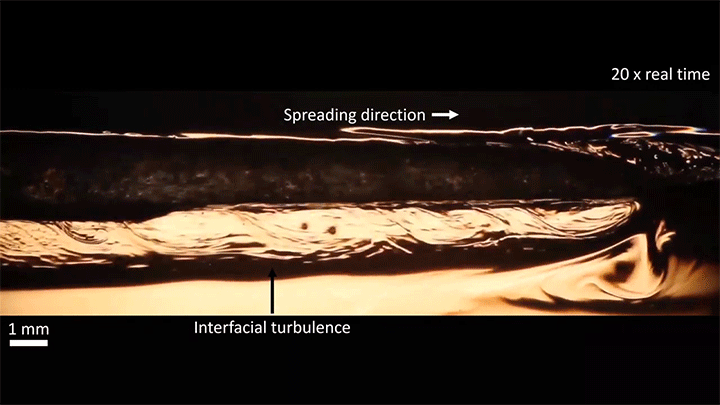
Once detached, the droplet takes on a flattened, lens-like shape that continues to spread. That spreading is driven by the mixing of ethanol and water, which generates heat and, thus, convection around the drop. This not only spreads the droplet, it causes turbulent behavior along the drop’s interface. (Image and video credit: S. Yamanidouzisorkhabi et al.)