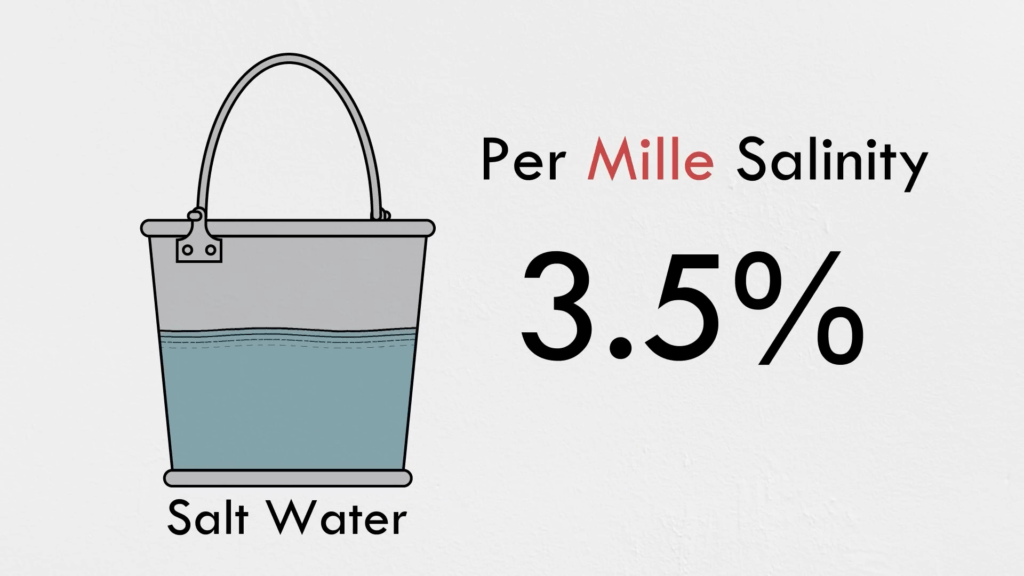
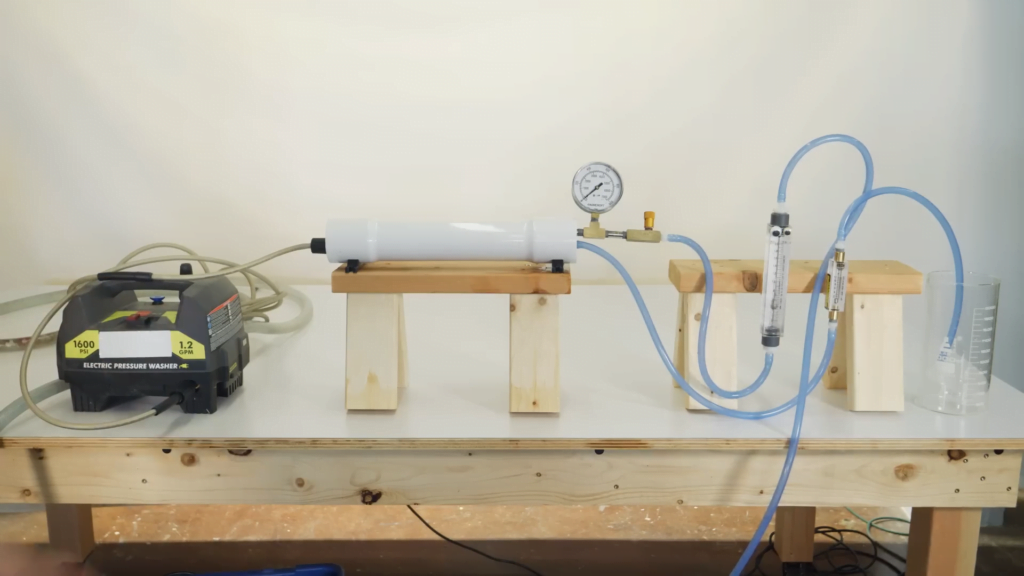
Desalination — the removal of salt from water — is an important process for providing the fresh water we need, but it’s quite expensive in terms of energy. In this Practical Engineering video, Grady demonstrates small-scale versions of the two most common methods for purifying water: distillation and reverse osmosis.
In distillation, salt water is boiled to separate the water into vapor that’s then condensed into freshwater. As straightforward as that sounds, though, the process is expensive, requiring a lot of energy for relatively little (albeit extremely pure) water. In contrast, reverse osmosis produces a somewhat less pure product at a lower energy cost. But it also produces brine, an even-saltier water that must be disposed of. (Video and image credit: Practical Engineering)