When placed on a vibrating oil bath, droplets have many wild behaviors, some of which mirror quantum mechanics. Even big droplets — bigger than 2 millimeters in diameter — can get in on the fun. This video shows several of these “jumbo superwalkers” in action, both singly and in groups. (Video and image credit: Y. Li and R. Valani; via GFM)
Search results for: “droplet”

Droplets Through a Forest
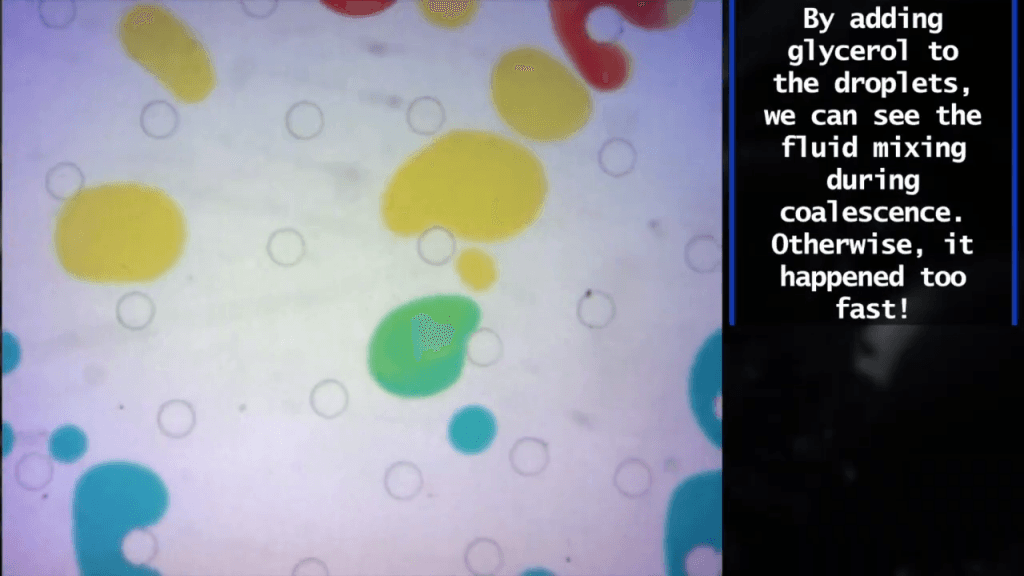
When droplets flow through a forest of microfluidic posts, they can deform around the obstacle or break up into smaller droplets. Here, researchers explore the factors that control the outcome, as well as when droplets collide, coalesce, and mix. (Video and image credit: D. Meer et al.)

Dancing Metal Droplets
Droplets of a gallium alloy are liquid at room temperature. When spiked with aluminum grains and immersed in a solution of NaOH, the droplets change shape and move in a random fashion. This video delves into the phenomenon, describing how a chemical reaction with the aluminum grains changes the local surface tension and creates Marangoni flows that make the droplets move. To get the droplet motion, you need to have the aluminum concentration just right. With too little, there’s not enough Marangoni flow. With too much, the hydrogen gas produced in the chemical reaction disrupts the droplet motion. (Video and image credit: N. Kim)

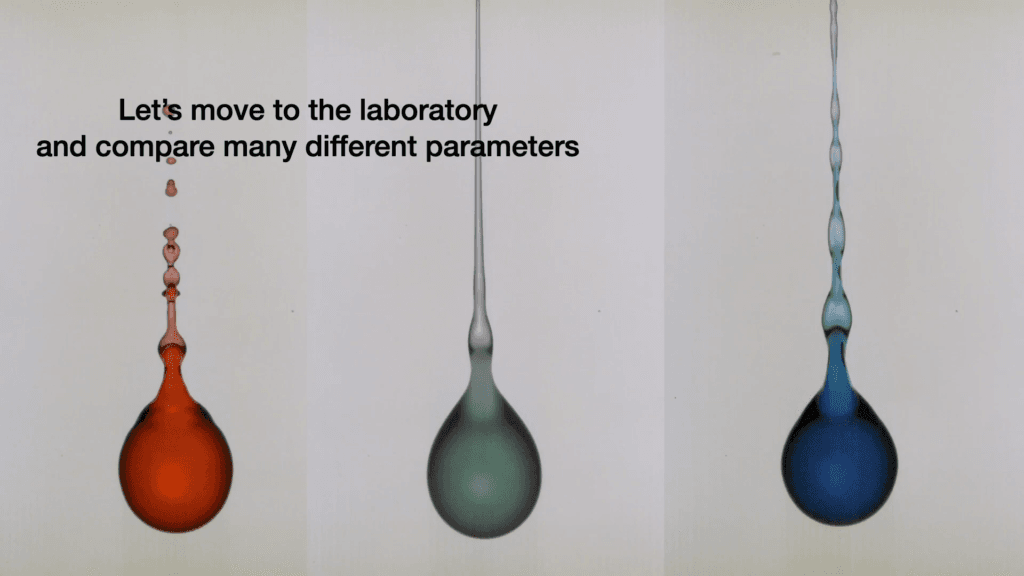
“Droplet on a Plucked Wire”
What happens to a droplet hanging on a wire when the wire gets plucked? That’s the fundamental question behind this video, which shows the effects of wire speed, viscosity, and viscoelasticity on a drop’s detachment. With lovely high-speed video and close-up views, you get to appreciate even subtle differences between each drop. Capillary waves, viscoelastic waves, and Plateau-Rayleigh instabilities abound! (Video and image credit: D. Maity et al.)

Hot Droplets Bounce
In the Leidenfrost effect, room-temperature droplets bounce and skitter off a surface much hotter than the drop’s boiling point. With those droplets, a layer of vapor cushions them and insulates them from the hot surface. In today’s study, researchers instead used hot or burning drops (above) and observed how they impact a room-temperature surface. While room-temperature droplets hit and stuck (below), hot and burning droplets bounced (above).
In this case, the cushioning air layer doesn’t come from vaporization. Instead, the bottom of the falling drop cools faster than the rest of it, increasing the local surface tension. That increase in surface tension creates a Marangoni flow that pulls fluid down along the edges of the drop. That flow drags nearby air with it, creating the cushioning layer that lets the drop bounce. In this case, the authors called the phenomenon “self-lubricating bouncing.” (Image and research credit: Y. Liu et al.; via Ars Technica)


The Mystery of the Binary Droplet
What goes on inside an evaporating droplet made up of more than one fluid? This is a perennially fascinating question with lots of permutations. In this one, researchers observed water-poor spots forming around the edges of an evaporating drop, almost as if the two chemicals within the drop are physically separating from one another (scientifically speaking, “undergoing phase separation“). To find out if this was really the case, they put particles into the drop and observed their behavior as the drop evaporated. What they found is that this is a flow behavior, not a phase one. The high concentration of hexanediol near the edge of the drop changes the value of surface tension between the center and edge of the drop. And that change is non-monotonic, meaning that there’s a minimum in the surface tension partway along the drop’s radius. That surface tension minimum is what creates the separated regions of flow. (Video and image credit: P. Dekker et al.; research pre-print: C. Diddens et al.)

Predicting Droplet Sizes
Squeeze a bottle of cleaning spray, and the nozzle transforms a liquid jet into a spray of droplets. These droplets come in many sizes, and predicting them is difficult because the droplets’ size distribution depends on the details of how their parent liquid broke up. Shown above is a simplified experimental version of this, beginning with a jet of air striking a spherical water droplet on the far left. In less than 3 milliseconds, the droplet has flattened into a pancake shape. In another 4 milliseconds, the pancake has ballooned into a shape called a bag, made up of a thin, curved water sheet surrounded by a thicker rim. A mere 10 milliseconds after the jet and drop first meet, the liquid is now a spray of smaller droplets.
Researchers have found that the sizes of these final droplets depend on the balance between the airflow and the drop’s surface tension; these two factors determine how the drop breaks up, whether that’s rim first, bag first, or due to a collision between the bag and rim. (Image credit: I. Jackiw et al.; via APS Physics)

How Water Droplets Charge Up
Rubbing a balloon on your hair can build a significant electrical charge. Water droplets have the same issue when they slide across a hydrophobic, electrically-insulated surface. A new study models why these charges build up and tests the model both experimentally and through simulation. They focused their theory on three effects that determine how much charge builds up. The first is a two-way chemical reaction that continuously creates charge at the interface, with positive charge building in the drop. Secondly, the drop’s contact angle with the surface sets how many protons can build up at the contact line, thereby affecting the electrical field they generate. And, finally, fluid motion at the rear of the drop deflects protons upward, shifting the electrical field. In particular, their model predicts that the higher contact angles of hydrophobic surfaces should increase charge build-up and faster sliding velocities should slow charge build-up, both of which agree with experiments.
The model should help researchers understand various charging scenarios, like those found on self-cleaning surfaces, in inkjet printing, and in semiconductor manufacturing. In the last scenario, rinsing semiconductor wafers in ultrapure water can build up charges in the kilovolt range, which is enough to damage the product. (Image credit: D. Carlson; research credit: A. Ratschow et al.; via APS Physics)